Question #d5404
1 Answer
Oct 26, 2015
For ionic bonds: You should familiarize yourself with the common oxidation states of the elements.
For covalent bonds: Know the number of valence electrons.
Explanation:
There really is no shortcut.
For ionic bonds (which is mostly a bond between metal and nonmetal), you can either memorize the most common oxidation states or you can utilize the table below as your guide.
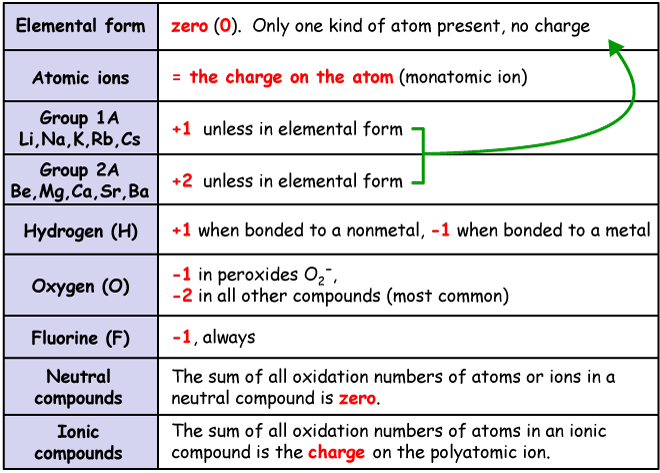 http://www.drcruzan.com/OxidationNumbers.html
http://www.drcruzan.com/OxidationNumbers.html
The rule for ionic bonds is to "exchange the superscripts for the subscripts".
For example:
For covalent bonds, it's a bit tricky since you have to know the number of valence electrons plus the elements can share at most six electrons between them.

