How would I determine the correct Lewis structure for NO3- (or any compound)? is it okay for the central atom to have a lone pair? I chose the structure that showed this (in a multi-choice question). But the correct answer showed N and O with a double bon
1 Answer
Normally, the first element in the chemical formula is the central atom, with the exception, of course, of hydrogen.
Explanation:
Say you are given the chemical formula
Except if the first element mentioned in the formula is
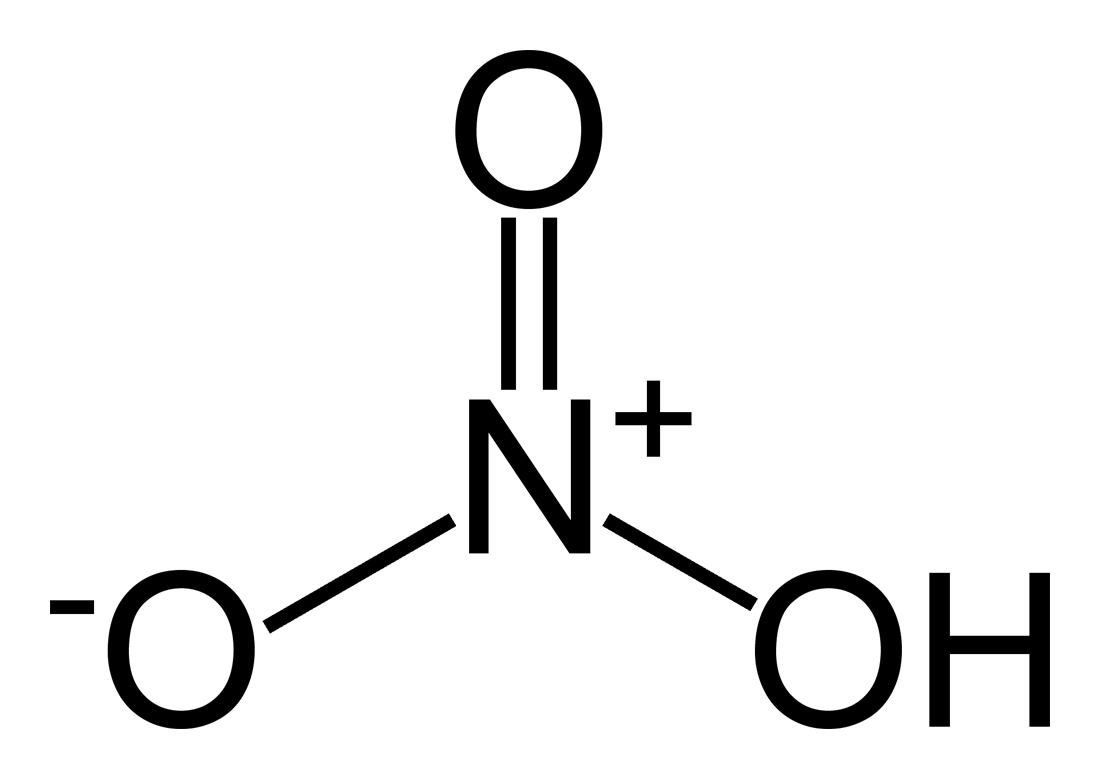
You have to remember that atoms like to have a total of eight valence electrons in order to be truly stable. With hydrogen, this isn't possible as it can only accommodate maximum of two electrons in its orbitals.
The "rule of eight" or Octet Rule is also the reason why there should be a double bond between the
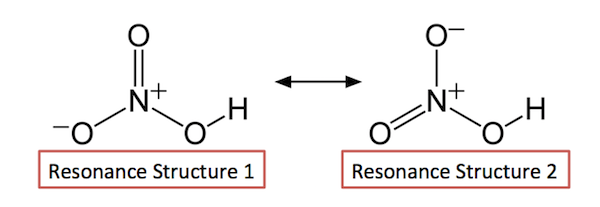
Please bear in mind that both structures are considered correct due to resonance or the delocalization of electrons in covalent bonds.

