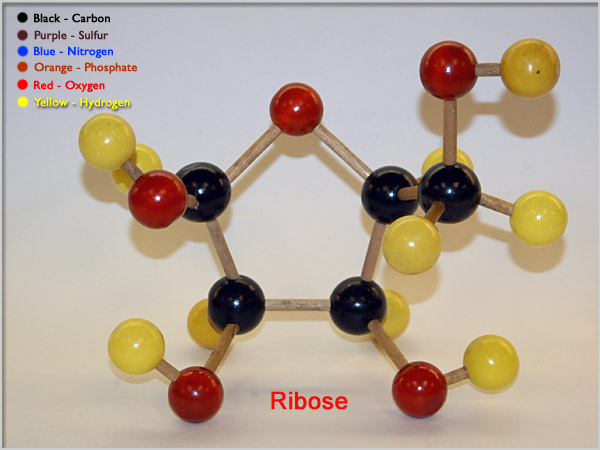
Ribose is an important sugar in RNA, with a molar mass of 150.15 g/mol. If its empirical formula is CH2O, what is its molecular formula?
1 Answer
Explanation:
The idea here is that the empirical formula tells you what the smallest whole number ratio that exists between the atoms of the elements that make up the compound is.
The molecular formula tells you the exact number of atoms of each element you need in order to form the molecule of the compound.
In essence, the empirical formual is like a building block that you must use in order to build the molecule.
In your case, the empirical formula of the compound,
- one carbon atom
- two hydrogen atoms
- one oxygen atom
So, what is the molar amss of a single building block? Use the molar mass of each individual atom
#overbrace(1 xx "12.0107 g/mol")^(color(blue)("one carbon atom")) + overbrace(2 xx "1.00794 g/mol")^(color(orange)("two hydrogen atoms")) + overbrace(1 xx "15.9994 g/mol")^(color(red)("one oxygen atom")) = "30.0260 g/mol"#
How many building blocks will you need to get the molar mass of the molecule?
#("30.0260 g/mol") * color(blue)(n) = "150.15 g/mol"#
#color(blue)(n) = (150.15color(red)(cancel(color(black)("g/mol"))))/(30.0260color(red)(cancel(color(black)("g/mol")))) = 5.00067 ~~ 5#
This means that the molecular formula of ribose will be
#("CH"_2"O")_color(blue)(5) = "C"_5"H"_10"O"_5#
Here's how the ribose molecule looks like