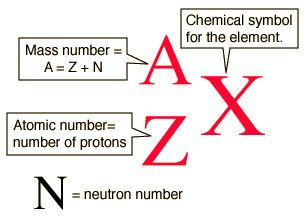What is the mass number of oxygen?
2 Answers
The most common Isotope Mass for Oxygen is 16 Atomic Mass Units.
Explanation:
The Weighted Average Atomic Mass for each element is found on the periodic table (That long decimal number near the bottom of each box). This number is a combination of all the known Isotopes and basically tells you the average mass you would expect per atom if you grabbed a random handful of them.
The Mass Number is the mass of a particular atom individually and is measured in Atomic Mass Units (AMU); these will always be whole numbers.
The most common Isotope is found by rounding the Weighted average found on the periodic table to the nearest whole number.
In this case Oxygen has a Weighted Average Atomic Mass of 15.999 AMU. So the Mass Number is 16.
That depends on the isotope of oxygen.
Explanation:
Mass number
The most common isotope of oxygen has 8 neutrons. Its atomic number is 8.
This isotope of oxygen is oxygen-16.
Another isotope of oxygen has 9 neutrons.
This isotope is oxygen-17.
A third oxygen isotope has 10 neutrons.
This oxygen isotope is oxygen-18.
The following is the isotopic or nuclear notation of an isotope.