How does ionization energy change across a period and down a group?
2 Answers
Ionization energies increase across a Period, and decrease down a Group.
Explanation:
The first ionization energy is the energy required to produce a mole of gaseous ions and a mole of gaseous electrons from a mole of gaseous atoms.
As we go from right to left across a period, the nuclear charge increases sequentially, while atomic radius decreases as electrons (in the same shell), are are held closer to the nucleus. It should therefore be harder to remove an electron from an atom further to the right on the period; and indeed it is (mind you, as a physical scientist, you should look up a table of the ionization energies).
As we descend a row (a column) on the Periodic table, the outermost electrons are further removed from the nucleus. Ionization energies should therefore decrease down a row of the Periodic table.
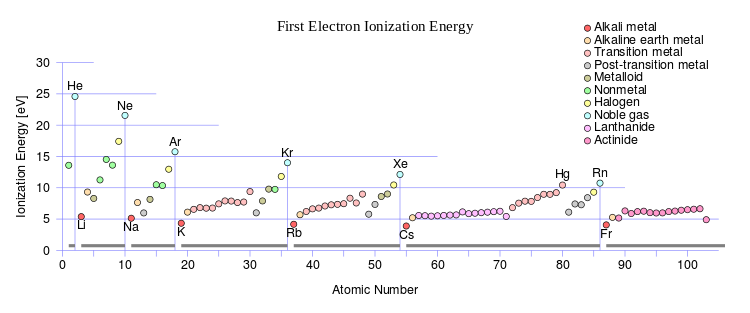
Does the wiki graph (source) illustrate what was earlier proposed? This graph should mirror the decrease in atomic radii.
Ionization energies decrease DOWN a Group, but increase across a PERIOD (from left to right AS WE FACE THE TABLE).
Explanation:
Two properties are important in determining ionization energies: (i) nuclear charge; and (ii) shielding by other electrons.
In partially filled shells, electrons shield each other from the nuclear charge very imperfectly, so across the Period (from left to right) as the nuclear charge,
On the other hand, down a Group, the increased nuclear charge is effectively shielded by the filled electronic shells. Nucleus/valence electron attraction becomes attenuated, and ionization energies decrease.
As a chemist, as a physical scientist, you should look at some tables of ionization energies and atomic radii, and see if what I have said is reasonable.

