How do you measure the decay of radioactive isotopes?
1 Answer
Mass-spectrometry
Explanation:
This is a huge topic, one that you can endless textbooks on and one that progressed hugely in the last 20-30 years. I will give you a very short run down of one way to measure isotopes from radioactive decay.
We will use a zircon (
But, zircon will also take in some trace elements, these trace elements will be present in the crystal at concentrations in the ball park of parts per million (ppm), which is a very small amount.
Zircon likes to take in the element uranium (
U-238 -------> Pb-206
U-235 -------> Pb-207
So two different isotopes of uranium will decay to two different isotopes of lead. So now our zircon has crystallised it has locked in the amount of uranium and lead it has taken in and now the uranium will be begin decaying to lead.
What we do next is measure all of these isotopes on a mass-spectrometer (something like the SHRIMP) and then say to ourselves, if we have this much lead and then we must have had this much uranium ( because uranium decays to lead). We know how long it takes for a given amount of uranium to decay to lead and so we can back calculate.
We can make a plot like the one below:
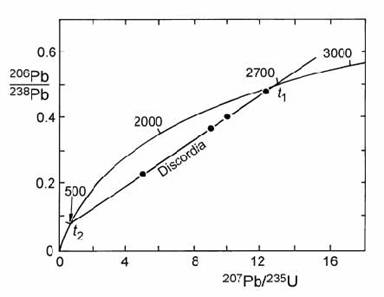
Imagine the black dots are our analyses, we can draw a straight line through them and where the line intercepts the curve it will define an age.
These are the real basics of it, it gets much more complicated and there are also limitations to this technique which I will not go into as the response is already too long! But if you're interested just search: U-Pb age dating and you can get some extra information.