How does heat capacity change with temperature?
1 Answer
It depends on the compound. It tends to increase as temperature increases.
For example, methane gas has a heat capacity equation:
where
So if you plug in these values for a specific temperature, you get the heat capacity. If you plot this for multiple temperatures, you get essentially a hyperbolic curve.
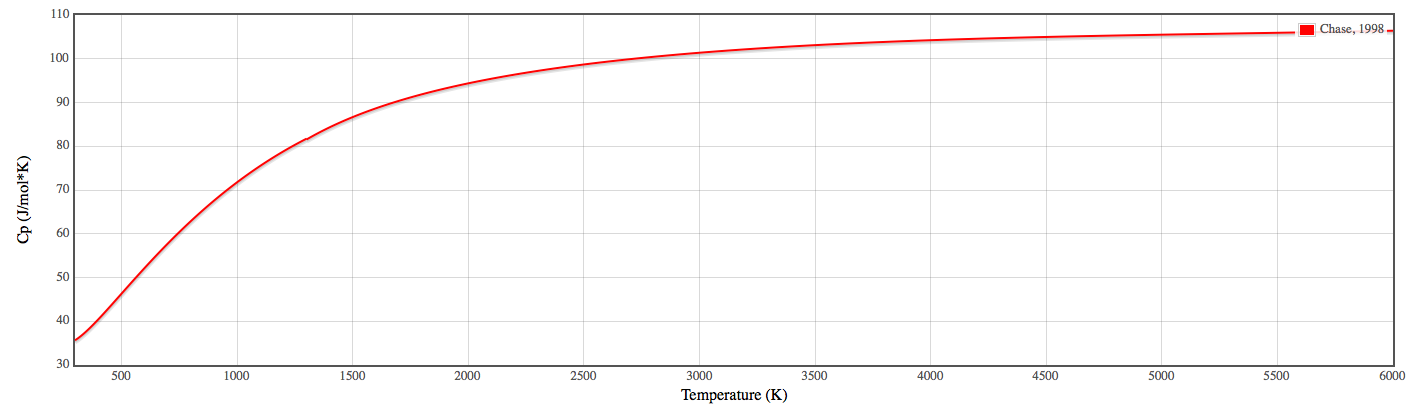
So, over
For a liquid, as you can imagine, the change is less drastic because the entropy is much lower for a liquid than a gas, or for a solid than a liquid.

