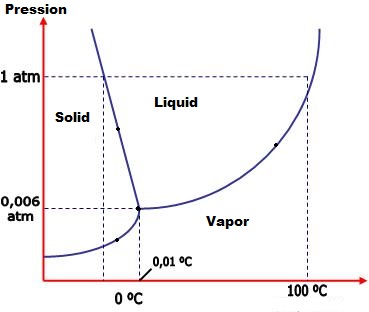
What two factors determine the point at which a liquid will boil?
1 Answer
Dec 29, 2015
The temperature and the pressure.
Explanation:
As we heat or/and increase the pressure in one substance, we are increasing the kinetic energy of its molecules. When the kinetic energy hits a certain level, the intermolecular forces aren't strong enough to hold it in its phase, and then the substance changes its phase. Each substance has a phase diagram for each phase change, like this - the water phase diagram: