Question #a409b
1 Answer
The answer is correct.
Explanation:
Cracking is a process used to convert complex organic molecules like long - chain hydrocarbons into simpler molecules by braking carbon - carbon bonds.
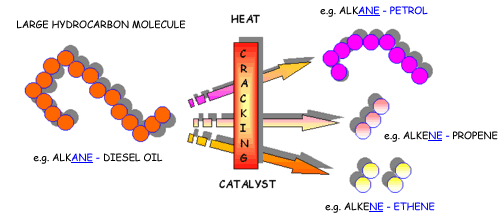
The most important thing to remember about cracking is that, like any chemical reaction, it must follow the principle of mass conservation.
That is, the number of atoms present in the initial molecule must be equal to the number of atoms present in the resulting molecules.
This means that any cracking reaction that results in the formation of products that have fewer atoms than the initial molecule will be incorrect.
Likewise, any cracking reaction that results in the formation of products that have more atoms than the initial molecule will not be accurate.
So, nonane,
- nine atoms of carbon
- twenty atoms of hydrogen
This means that the products of the cracking reaction must contain a total of nine atoms of carbon and twenty atoms of hydrogen.
The product listed as an answer,
- ten atoms of carbon
- twenty-two atoms of hydrogen
Therefore, this is not a possible product for the cracking of nonane.

