How do you write the formula for calcium fluoride?
1 Answer
Jan 12, 2016
The formula for calcium fluoride is
Explanation:
An ionic compound is neutral, which means its overall charge is zero. Therefore, the sum of the charges of each ion in the compound must equal zero.
In calcium fluoride, the calcium ion
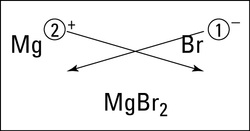
There is a method that makes it fairly easy to determine the formula unit of an ionic compound. It's called the crisscross method. In the crisscross method, the size of the charge of each ion becomes the subscript of the other ion.