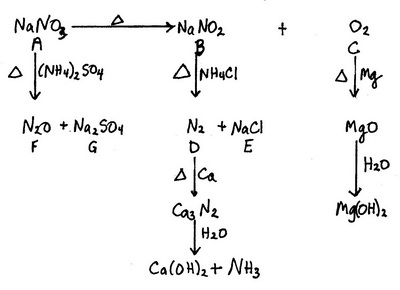
All the credit for this answer goes to Stefan V.
The big clues are the yellow flame colour (#"Na"#) and the brown gas (#"NO"_2# or, less likely, #"Br"_2#).
#"NO"_2# comes from the decomposition of #"HNO"_2#.
This implies #"NaNO"_2# and, along with other evidence, #"NaNO"_3#.
#color(red)(bb"A" = "NaNO"_3)#
#2underbrace("NaNO"_3)_color(red)("A") stackrelcolor(blue)(Δcolor(white)(X)) (→) 2underbrace("NaNO"_2)_color(red)("B") + underbrace("O"_2)_color(red)("C")#
#color(red)(bb"C" = "O"_2)#
#"O"_2# is a colourless gas that forms #"MgO"# when heated with #"Mg"#.
#"O"_2 + "2Mg" → "2MgO"#
#"MgO"# is a basic oxide.
#"MgO" + "H"_2"O" ⇌ "Mg"^(2+) + "2OH"^(-)#
#color(red)(bb"B" = "NaNO"_2)#
#"NaNO"_2# reacts with sulfuric acid to form #"HNO"_2#, which decomposes to form the brown gas #"NO"_2#.
#2underbrace("NaNO"_2)_color(red)("B") + "H"_2"SO"_4 → "2HNO"_2 + "Na"_2"SO"_4#
#"2HNO"_2 → underbrace("NO"_2)_color(red)("brown") + "NO" + "H"_2"O"#
#color(red)(bb"D" = "N"_2)# and #color(red)(bb"E" = "NaCl")#
#"NaNO"_2# reacts with #"NH"_4"Cl"# to form #"N"_2# and #"NaCl"#.
#underbrace("NaNO"_2)_color(red)("B") + "NH"_4"Cl" → underbrace("N"_2)_color(red)("D") + underbrace("NaCl")_color(red)("E") + "2H"_2"O"#
#"N"_2# reacts with #"Ca"# to form #"Ca"_3"N"_2#.
#"3Ca" + "N"_2 stackrelcolor(blue)(Δcolor(white)(X))(→) "Ca"_3"N"_2#
#"Ca"_3"N"_2# reacts with water to form ammonia and #"Ca"("OH")_2#.
#"Ca"_3"N"_2 + 6"H"_2"O" → 3underbrace("Ca"("OH")_2)_color(red)("basic") + 2underbrace("NH"_3)_color(red)("colourless") #
#color(red)(bb"F" = "N"_2"O")# and #color(red)(bb"G" = "Na"_2"SO"_4")#
#"NaNO"_3# reacts with #("NH"_4)_2"SO"_4# to form #"N"_2"O"# and #"Na"_2"SO"_4#
#2underbrace("NaNO"_3)_color(red)("A") + ("NH"_4)_2"SO"_4 stackrelcolor(blue)(Δcolor(white)(X))(→) 2underbrace("N"_2"O")_color(red)("F") + underbrace("Na"_2"SO"_4)_color(red)("G") + "4H"_2"O"#
The #"Na"# in #"Na"_2"SO"_4# gives the yellow flame colour.