How would you explain an ethyl group?
1 Answer
Its an alkyl derivative of ethane.
Explanation:
Alkanes have the formula
Alkanes have their carbons saturated with hydrogens and have no double bonds. An example is ethane.
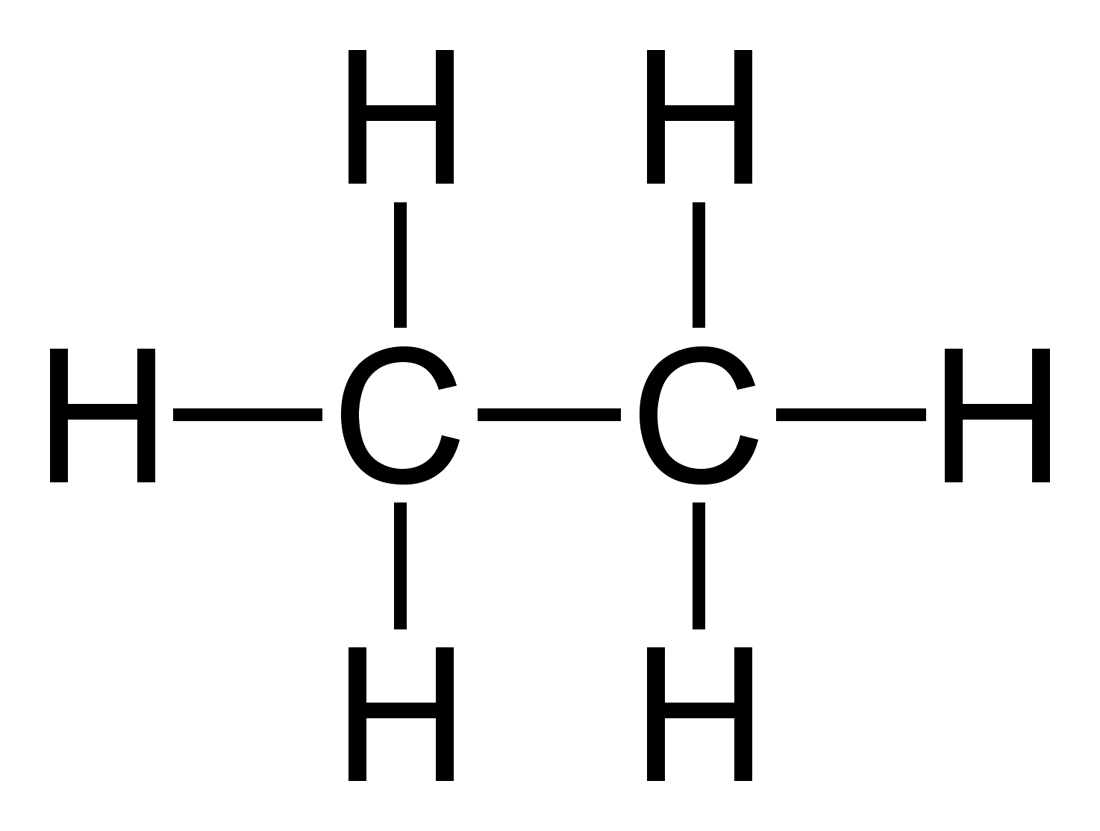 fr.academic.ru
fr.academic.ru
Image Source: 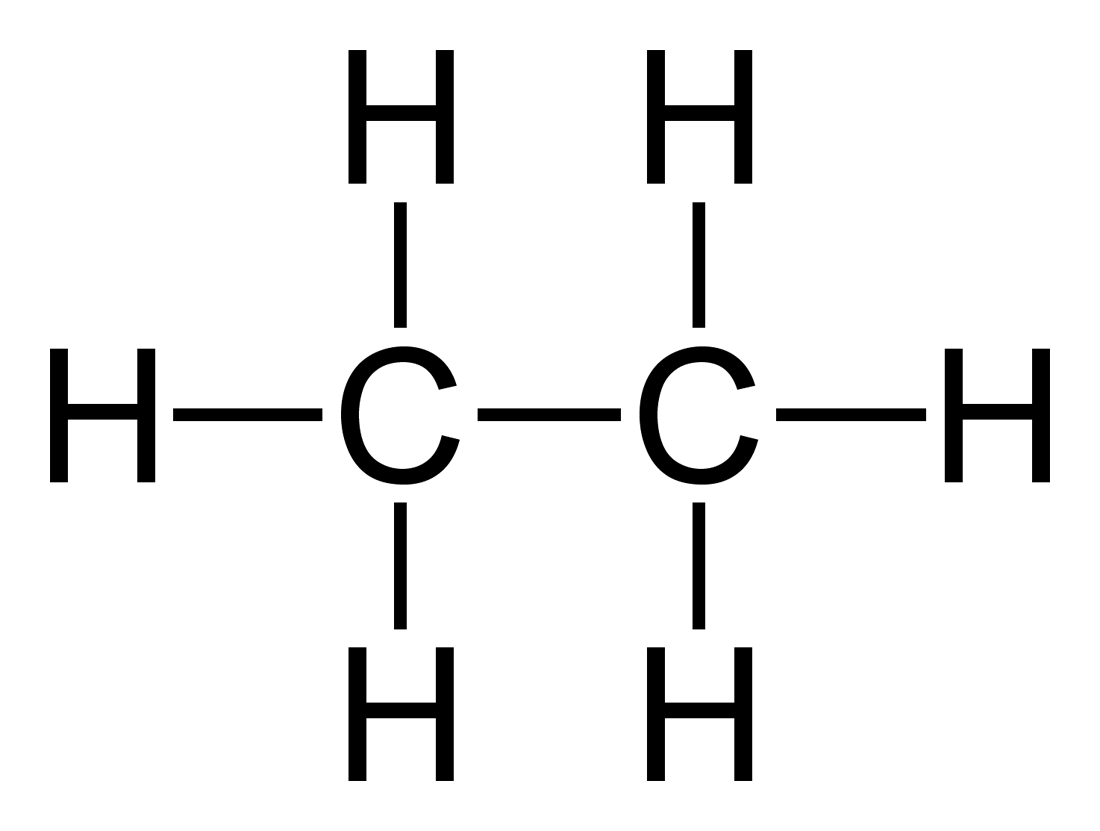 useruploads.socratic.org
useruploads.socratic.org
Now let us have a look in alkyl groups.
Alkyl groups are organic groups(they usually act as substituents) that are alkanes lacking one carbon and hence,
Take a look at the structure given above. Remove 1 hydrogen from
Remember that carbons have to be tetravalent. That is, its valence electrons need 4 electrons to bond covalently (see Octet rule). However, because we removed 1 Hydrogen atom, there is one valence electron that has no partner.
So some atoms or groups may bind to that missing valence electron. For example, i chose to put a hydroxyl group (OH-) to satisfy the missing valence electron. The resultant compound is now
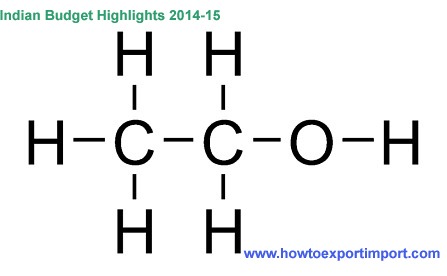 howtoexportimport.com
howtoexportimport.com
Image Source:  useruploads.socratic.org
useruploads.socratic.org

