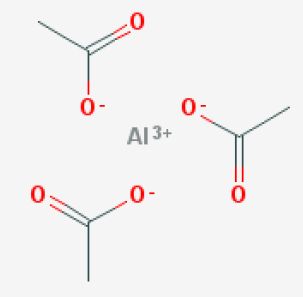
What is the total number of moles of atoms represented by the formula #Al(C_2H_3O_2)_3#?
1 Answer
Explanation:
You're dealing with an ionic compound, which means that you can start by looking for the number of moles of ions that make up one mole of formula units.
More specifically, this compound is known as aluminium triacetate, which means that it contains the aluminium cation,
Notice that you need three acetate anions in order to balance the
So, one mole of aluminium triacetate will contain
#1# mole of aluminium cations,#"Al"^(3+)# #3# moles of acetate anions,#3 xx "CH"_3"COO"^(-)#
Now focus on finding how many atoms make up each ion. In the case of the cation, you get one aluminium atom.
The acetate anion is a polyatomic ion, which means that every mole of acetate anions will contain
#2# moles of carbon atoms,#2 xx "C"# #3# moles of hydrogen atoms ,#3 xx "H"# #2# moles of oxygen atoms,#2 xx "O"#
Since the compound contains three moles of acetate anions, you will get a total of
#3 xx overbrace((2 xx "C"))^(color(blue)("1 acetate ion")) = 6 xx "C" -># six moles of carbon atoms
#3 xx overbrace((3 xx "H"))^(color(blue)("1 acetate ion")) = 9 xx "H" -># nine moles of hydrogen atoms
#3 xx overbrace((2 xx "O"))^(color(blue)("1 acetate ion")) = 6 xx "H" -># six moles of oxygen atoms
So, the total number of moles of atoms found in one mole of aluminium triacetate will be
#overbrace(1 xx "Al")^(color(red)(1 xx "Al"^(3+))) + overbrace(6 xx "C" + 9 xx "H" + 6 xx "O")^(color(purple)(3 xx "CH"_3"COO"^(-))) = color(green)(22)#