Question #e1c0b
1 Answer
You can calculate it using Hess' Law.
Explanation:
Hess' Law states that the overall enthalpy change for a reaction is independent of the route taken.
We can apply this principle when calculating enthalpies of reaction which are not easy to measure directly, as is the case here.
Since tetrazene and hydrazine are made up of the same elements you start your cycle by writing down the equation for the reaction you are interested in.
Underneath you add the elements and then complete the cycle as indicated:
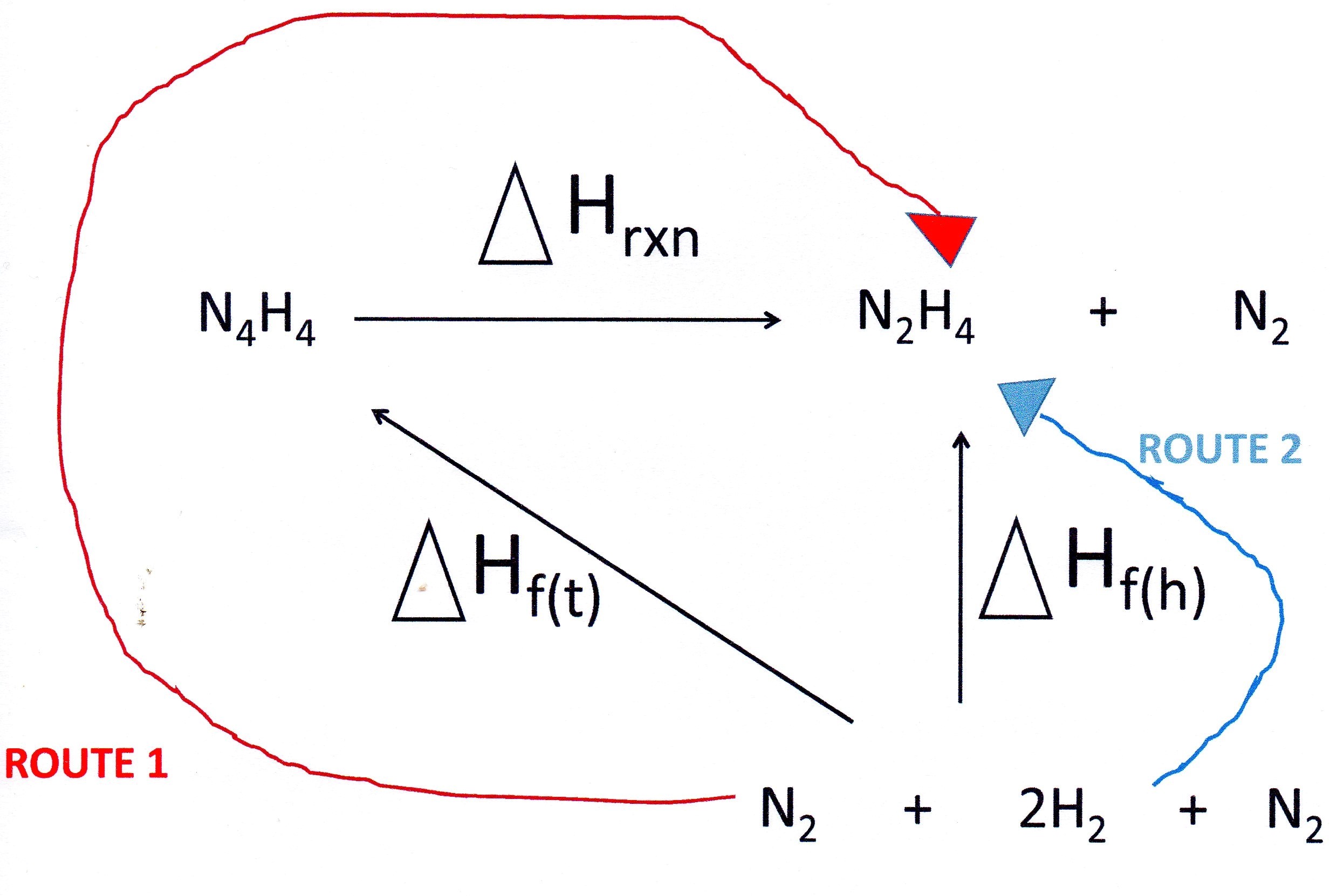
So we can write:
I looked up the enthalpies of formation in a date table:
Note that I did not include the enthalpy of formation of nitrogen in the calculation since it is an element and, by definition, is equal to zero.
A more general form of this calculation is :
Try and remember that this comes from Hess' Law.

