Is the ortho isomer necessarily more acidic than the para isomer?
1 Answer
You mean for disubstituted benzene compounds? In general it is true, but sometimes there are exceptions, so be careful. o-nitrophenol has about the same pKa than p-nitrophenol (http://www.chem.ucla.edu/~cantrill/30A_S05/Acids_Bases_2_Ans.pdf).
Generally it would mean the resonance structure of the conjugate base stabilizes the para isomer more (= more weakly acidic or more strongly basic), so the original para isomer is less acidic.
Let's examine the conjugate base of o-nitroaniline and compare it to that of p-nitroaniline.
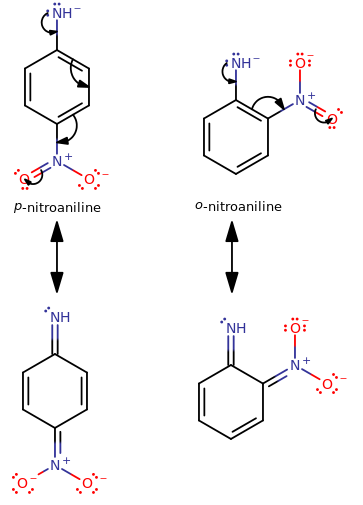
You should notice that the
The greater delocalization of
Additionally, since both substituents are acting in the same resultant direction (
Since this means the para isomer conjugate base is more stable (a stronger base) than the ortho isomer conjugate base, the original para isomer is a weaker acid than the original ortho isomer.
Therefore, the original ortho isomer is a bit more acidic. We can see that in the pKas:
#"pKa"_"ortho" ~~ -0.28#
(https://pubchem.ncbi.nlm.nih.gov/compound/2-nitroaniline)
#"pKa"_"para" ~~ 1.01#
(https://pubchem.ncbi.nlm.nih.gov/compound/7475)
(A lower

