When using the Nernst equation when can it be directly express in terms of concentrations or activities?
1 Answer
Conentrations can be used accurately only at low vales.
Explanation:
The Nernst Equation is:

The more concentrated a solution becomes the more the ions tend to loosely bind as "ion pairs". This reduces their "effective concentration".
The activity is a measure of this effective concentration. the relationship between activity and concentration is given by:
At low concentrations the value of
The graphic shows how the red observed potential line deviates from the blue Nernst predicted line as the concentration increases.
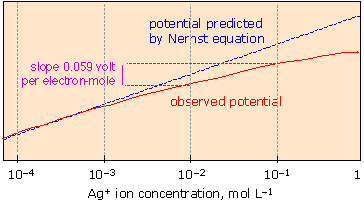
The Nernst Equation cannot accurately predict cell potential if the total ion concentration starts to exceed ~
Ion activitiy coefficients can be measured electrochemically and these can then be used to calculate activities if you are working at higher concentrations.

