What is the mass number for Technetium when the atom has 56 neutrons?
1 Answer
Feb 6, 2016
The mass number of this isotope of technetium is 99.
Explanation:
The mass number, A, of an isotope is the number of protons (Z) and neutrons (N) in the nuclei of its atoms.
Technetium (Tc) has atomic number, Z= 43, which means that all of its atoms have 43 protons in their nuclei.
To determine the mass number of this isotope of technetium, add the protons and the neutrons.
This isotope of Tc is technetium-99. Its nuclear notation (isotopic notation) is:
Generic Nuclear Notation
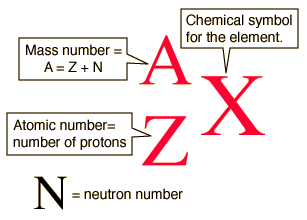 http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html
http://hyperphysics.phy-astr.gsu.edu/hbase/nuclear/nucnot.html

