Question #c0a6f
1 Answer
In covalent bonding Non metals combine together by sharing electrons. The shared pair of electrons holds the two atoms together. It's called a covalent bond. The group of atoms bonded together in this way is called a molecule.
It occurs because of the tendency of the atom to have its valence shell full (
This is one of an example of how an atom shares its electrons with the other element in such a way that the two elements have full
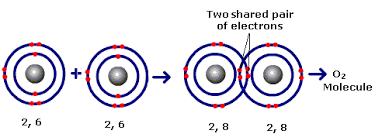
In the above example the


