Why is this not possible? 2Cl2 + 4NaOH -> 4NaCl + 2H20 + O2
2Cl2 + 4NaOH -> 4NaCl + 2H20 + O2
Why isn't this reaction possible? Because the oxygen in [OH] will have to undergo oxidation from [-2] to [zero], and there's no powerful oxidizer around?
The reaction from the textbook is:
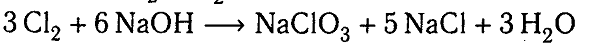
I tried to come up with an alternative reaction with no NaClO3 arising.
2Cl2 + 4NaOH -> 4NaCl + 2H20 + O2
Why isn't this reaction possible? Because the oxygen in [OH] will have to undergo oxidation from [-2] to [zero], and there's no powerful oxidizer around?
The reaction from the textbook is:
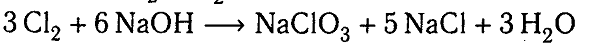
I tried to come up with an alternative reaction with no NaClO3 arising.
1 Answer
#2"Cl"_2 + 4"NaOH" -> 4"NaCl" + 2"H"_2"O" + "O"_2#
First, let's assume we don't know the products. At that point we should know that chlorine gas is reacting in base. If that is the case, it is not a simple acid/base reaction, because there is no acid.
That, along with the single-replacement form of the reaction, suggests that it is a redox reaction (like you know), and we can't immediately expect that we would make an equimolar amount of
(Perhaps you put
Another way you could look at it is to notice that oxygen changed oxidation state from
That means that somehow, you had oxygen get... oxidized. More often than not, oxygen or oxygen-containing compounds (like oxohalogen anions, or peroxides) would oxidize, not get oxidized.
WHEN IN DOUBT, USE YOUR TEXTBOOK REFERENCE TABLES
Now, I would start looking at what kinds of redox reactions in base (or not in acid) that
#"Cl"_2(g) + 2e^(-) -> 2"Cl"^(-)# (you could have predicted this one)
But that doesn't utilize
Two of the reactions in the reference table qualify, but since
#"Cl"^(-)(aq) + 2"OH"^(-)(aq) -> "ClO"^(-)(aq) + "H"_2"O" + 2e^(-)#
#"Cl"^(-)(aq) + 6"OH"^(-)(aq) -> "ClO"_3^(-)(aq) + 3"H"_2"O" + 6e^(-)#
As you can see, neither of these yield
DETERMINING THE FINAL REACTION
Now let's suppose we went with the first option as a half reaction.
#"Cl"_2(g) + cancel(2e^(-)) -> 2"Cl"^(-)#
#"Cl"^(-)(aq) + 2"OH"^(-)(aq) -> "ClO"^(-)(aq) + "H"_2"O" + cancel(2e^(-))#
#"-------------------------------------------------"#
#"Cl"_2(g) + "Cl"^(-)(aq) + 2"OH"^(-)(aq) -> "ClO"^(-)(aq) + 2"Cl"^(-)(aq) + "H"_2"O"#
Now, our only cation is sodium, so if we add back the sodium spectator ions, we get:
#color(blue)("Cl"_2(g) + 2"NaOH"(aq) -> "NaClO"(aq) + "NaCl"(aq) + "H"_2"O")#
Here,
#3("Cl"_2(g) + cancel(2e^(-)) -> 2"Cl"^(-))#
#"Cl"^(-)(aq) + 6"OH"^(-)(aq) -> "ClO"_3^(-)(aq) + 3"H"_2"O" + cancel(6e^(-))#
#"-------------------------------------------------"#
#3"Cl"_2(g) + 6"OH"^(-)(aq) -> "ClO"_3^(-)(aq) + 5"Cl"^(-)(aq) + 3"H"_2"O"#
Add back the spectator sodium:
#color(blue)(3"Cl"_2(g) + 6"NaOH"(aq) -> "NaClO"_3(aq) + 5"NaCl"(aq) + 3"H"_2"O")#
Here,
Both of these are possible reactions. They are called disproportionation reactions.
So I'm guessing that you were given ahead of time that you are making

