At what temperature is the concentration of a saturated solution of #KCl# (molar mass 74.5 g) approximately 3 molal?
1 Answer
Explanation:
In order to be able to answer this question, you need to have the solubility graph of potassium chloride,
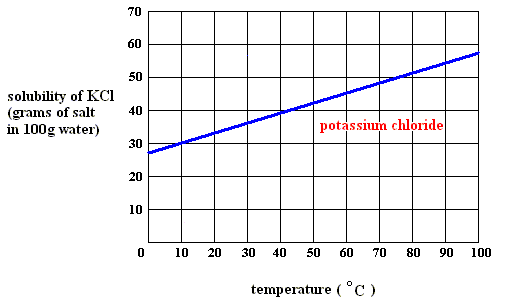
Since the solubility of potassium chloride is given per
Keep in mind that molality is defined as moles of solute, which in your case is potassium chloride, divided by kilograms** of solvent, which in your case is water.
#color(blue)(b = n_"solute"/m_"solvent")#
This means that you have
#n_"solute" = b * m_"solvent"#
#n_"solute" = "3.5 mol" color(red)(cancel(color(black)("kg"^(-1)))) * 100 * 10^(-3)color(red)(cancel(color(black)("kg"))) = "0.35 moles KCl"#
Next, use potassium chloride's molar mass to figure out how many grams would contain this many moles
#0.35color(red)(cancel(color(black)("moles KCl"))) * "74.5 g"/(1color(red)(cancel(color(black)("mole KCl")))) = "26.1 g"#
Now take a look at the solubility graph and decide at which temperature dissolving
Practically speaking, you're looking for the temperature at which the saturation line, drawn on the graph in
From the look of it, dissolving this much potassium chloride per

