If a chemist needs to make 35.05 g of CuI_2, how many mL of a 3.3 M solution of NaI must he use, assuming there is excess copper (II) nitrate?
1 Answer
This will be equal to 66.94 mL of 3.3 M NaI solution.
Explanation:
To answer this problem, determine the balanced chemical reaction involved which is shown below:
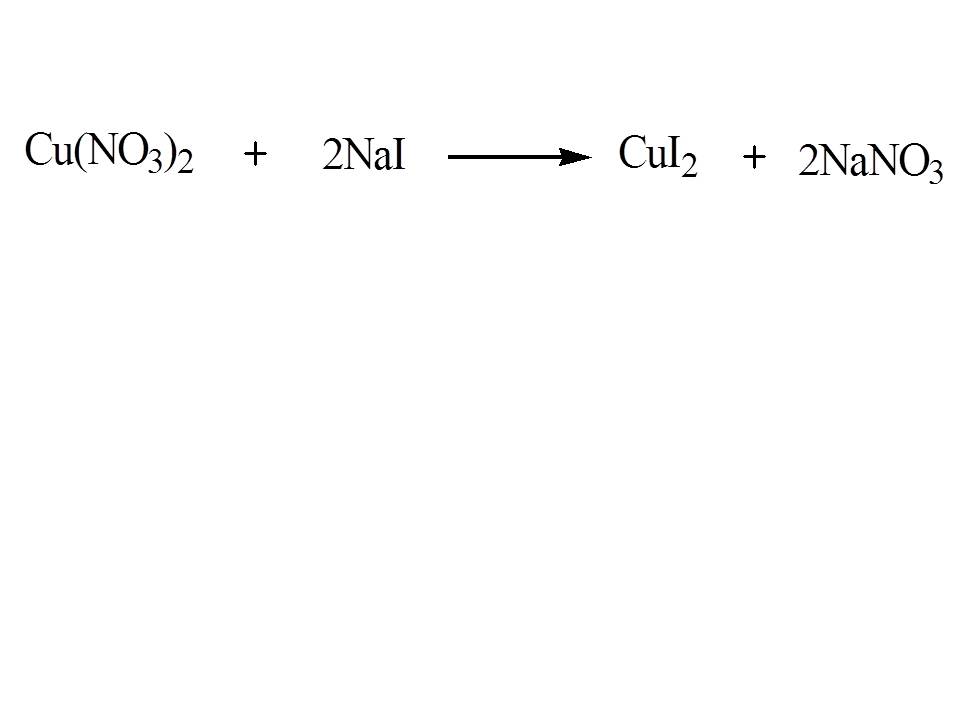
Using dimensional analysis, lets determine the number of moles of sodium iodide needed to produced 35.05 g of copper (II) iodide. An outline of the calculation will be first divide first the amount of copper (II) iodide with its multiply then multiply it with the appropriate dimension factor based on the balanced chemical reaction which will give you an answer equal to 0.2209 mol NaI:

Then, two determined the volume of NaI needed, will going to divide the calculated number of moles of NaI that was calculated with the concentration of NaI solution that was given in the answer. Converting liter to milliliters will give us a volume equal to 66.94 mL:
