How many grams of sugar would you need to add to water to make 235 grams of a 7% solution? using this formula: (Please Help!)
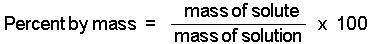
1 Answer
Explanation:
A solution's percent concentration by mass,
In your case, sugar is the solute and water is the solvent. A solution is formed when you dissolve a solute in a solvent.
Here's how to solve this problem without using the formula for percent concentration by mass, which is given to you as
#color(blue)("% w/w" = "mass of solute"/"mass of solution" xx 100)#
You know that your target sugar solution must be
This ratio between sugar and water is the same regardless of the mass of solution. In your case, you want the solution to have a mass of
#235 color(red)(cancel(color(black)("g solution"))) * overbrace("7 g sugar"/(100color(red)(cancel(color(black)("g solution")))))^(color(purple)("= 7% w/w")) = "16.45 g sugar"#
You should round this off to one sig fig, since that's how many sig figs you have for the percent by mass, but I'll leave it rounded to two sig figs, just for good measure
#m_"sugar" = color(green)("16 g")#
This is what the formula for percent concentration by mass actually means. If you start with
#"% w/w" = m_"solute"/m_"solution" xx 100#
you can rearrange to solve for
#"% w/w" * m_"solution" = m_"solute" * 100#
#m_"solute" = ("% w/w" * m_"solution")/100#
Now plug in your values to get
#m_"solute" = (7 * "235 g")/100 = color(green)("16 g")#

