Why and how do we get #"NH"_4"NO"_3# when reacting very dilute #"HNO"_3# with #"Zn"#?
From Russian Wikipedia's article on nitric acid:
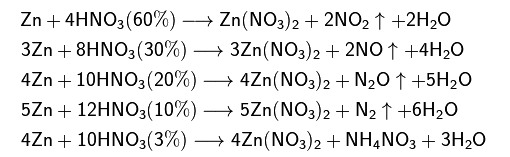
enter image description here
It's clear that as we decrease the concentration of nitric acid, we get more and more reduced nitrogen: in NO2, the oxidation state of N is (+4), then it goes to zero in N2 (when we use 10% strong HNO3).
But why all of a sudden we get NH4NO3? In NH4, the oxidation of N is (-3), in NO3, the oxidation state is (+5). So the overall oxidation increases by 2.
I wonder how this ammonium nitrate is generated. Where the ammonium ion evolves from.
From Russian Wikipedia's article on nitric acid:
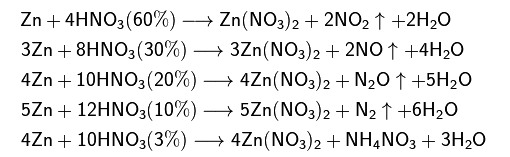
enter image description here
It's clear that as we decrease the concentration of nitric acid, we get more and more reduced nitrogen: in NO2, the oxidation state of N is (+4), then it goes to zero in N2 (when we use 10% strong HNO3).
But why all of a sudden we get NH4NO3? In NH4, the oxidation of N is (-3), in NO3, the oxidation state is (+5). So the overall oxidation increases by 2.
I wonder how this ammonium nitrate is generated. Where the ammonium ion evolves from.
1 Answer
You get ammonium nitrate when nitric acid is reduced to ammonia.
Explanation:
What your book doesn't tell you is that very dilute nitric acid will react with zinc to form ammonia,
Here's how you can think about what's going on. As you know, nitric acid is a strong oxidizing agent.
When you react metals with nitric acid, the reaction does not produce hydrogen gas. That happens because as soon as the gas is formed, it will be oxidized to water.
At the same time, the acid will be reduced. As you can see from your book, the more dilute the nitric acid, the greater the reduction.
This is exactly what happens with very dilute, and I think very cold nitric acid. Nitrogen will be reduced from an oxidation state of
Alternatively, you can see this as a reduction from
The first step in this reaction is
#"Zn"_text((s]) + 2"HNO"_text(3(aq]) -> "Zn"("NO"_3)_text(2(aq]) + "H"_text(2(g])#
However, hydrogen gas gets oxidized instantly
#color(blue)("H"_text(2(g]) -> 2"H"_text((aq])^(+) + 2"e"^(-))#
At this point, the acid is reduced to ammonia
#"HNO"_text(3(aq]) + color(red)(4) xx[color(blue)(2"H"_text((aq])^(+) + 2"e"^(-))] -> "NH"_text(3(aq]) + 3"H"_2"O"_text((l])#
Finally, ammonia is being neutralized by the acid to ammonium nitrate
#"NH"_text(3(aq]) + "HNO"_text(3(aq]) -> "NH"_4"NO"_text(3(aq])#
So, let's put all this together to get the overall reaction. Notice that you have a
#color(red)(4) xx ["Zn"_text((s]) + 2"HNO"_text(3(aq]) -> "Zn"("NO"_3)_text(2(aq]) + "H"_text(2(g])]#
#"HNO"_text(3(aq]) + color(red)(4) xx[color(blue)(2"H"_text((aq])^(+) + 2"e"^(-))] -> "NH"_text(3(aq]) + 3"H"_2"O"_text((l])#
#"NH"_text(3(aq]) + "HNO"_text(3(aq]) -> "NH"_4"NO"_text(3(aq])#
#color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)/color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)#
#4"Zn"_text((s]) + 8"HNO"_text(3(aq]) + "HNO"_text(3(aq]) + color(red)(cancel(color(black)(8"H"_text((aq])^(+) + 8"e"^(-)))) + color(purple)(cancel(color(black)("NH"_text(3(aq])))) + "HNO"_text(3(aq]) -> 4"Zn"("NO"_3)_text(2(aq]) + color(red)(cancel(color(black)(8"H"_text((aq])^(+) + 8"e"^(-)))) + color(purple)(cancel(color(black)("NH"_text(3(aq])))) + 3"H"_2"O"_text((l]) + "NH"_4"NO"_text(3(aq])#
This will get you
#color(green)(4"Zn"_text((s]) + 10"HNO"_text(3(aq]) -> 4"Zn"("NO"_3)_text(2(aq]) + "NH"_4"NO"_text(3(aq]) + 3"H"_2"O"_text((l]))#
And there you have it - very dilute and cold nitric acid reacts with zinc to produce ammonium nitrate.

