Question #86b5f
1 Answer
Explanation:
I'm not sure what you mean by "a lot of steps" because you only need one calculation to get the answer here. Basically, the answer is one step away.
I assume that your mole road map looks something like this
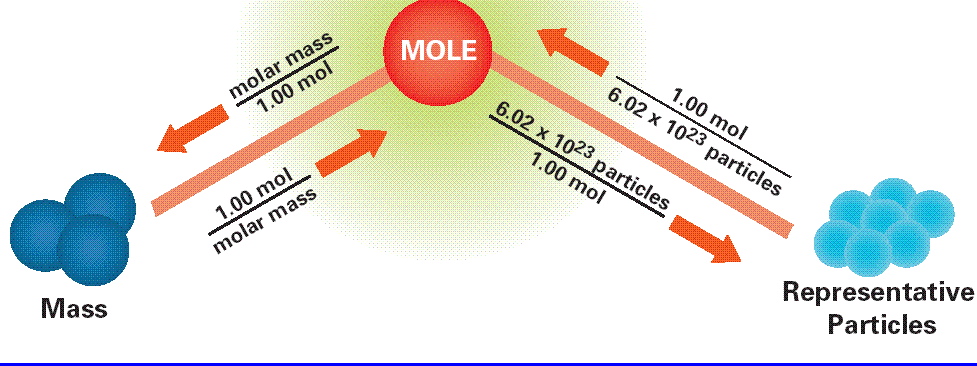
The problem wants you to convert moles of water to grams of water, which means that you're going to have to use the mole
The conversion factor that takes you from moles to mass and vice versa is called the molar mass.
The molar mass of a given compound is simply the mass of one mole of that compound. In your case, you must use the molar mass of water to determine how many grams would be equivalent to
Now, water has a molar mass of
If that's the case, then
#4.401 color(red)(cancel(color(black)("moles H"_2"O"))) * overbrace("18.015 g"/(1color(red)(cancel(color(black)("mole H"_2"O")))))^(color(purple)("molar mass of water")) = "79.284 g"#
You need to round this off to four sig figs, the number of sig figs you have for the moles of water
#m_(H_2O) = color(green)("79.28 g")#

