When does water vapor in air condense?
1 Answer
Depends on the availability of condensation nuclei, but generally at the dew point.
Explanation:
First of you need to understand that water molecules can be at different energy levels at many temperature. Therefore at any given temperature you can have water molecules of different states. This is because energy is not always distributed evenly between particles. At 0 degrees, the average temperature of the particles is 0 but individual molecules can have more or less energy.
At a given temperature there is a maximum amount of water vapor the air can hold.
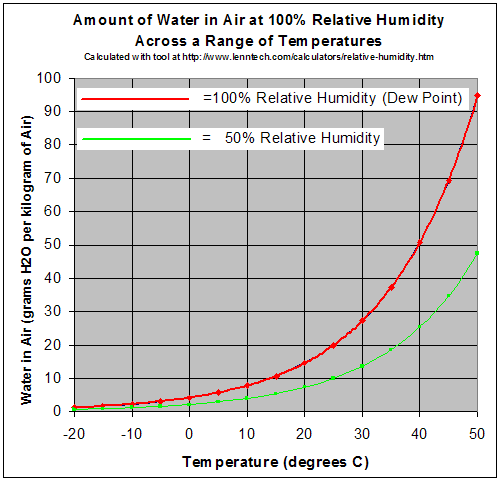
The red line equals the maximum amount of grams of water a kilogram of air can hold at a given temperature. So if you are adding water to air then once the number of grams per kilogram is reached, any additional water vapor will condense automatically. Conversely, if you have a constant amount of water in the air and you cool it, eventually it will reach a temperature where the amount of water is the maximum. At this point condensation occurs.
The temperature where the air is holding all the water it can is called the dew point.
Now what about condensation nuclei?
Water needs something to condense on in order for it to condense easily. This is usually a bit of dirt or salt (microscopic in size), and the water molecules adhere to it. If there is nothing for the water to condense on (conditions only really found in a lab) the vapor needs to spontaneously condense to liquid and that will only happen in super-saturated air (at around 300% relative humidity or 3 times the maximum amount of water vapor that the air is supposed to be able to hold).
Although air with no condensation nuclei does not really occur in nature there are situations where there are a lot of nuclei, and in those case we can have condensation occur at a lower relative humidity (so that the air might only be hold 70% of the maximum amount of water vapor and condensation still occurs). This is partly due to the amount of energy of individual particles and also due to the affinity for water of some types of condensation nuclei.
