Neither answer is correct. The first one should have a #2-# charge, and the last one should have a charge of #3+#.
FIRST PRODUCT
#["CoCl"_4]^(-)# is not right. What you have implied is that the cation is #"Co"^(3+)#, which has a coordination number of #6#, not #4#. #"Co"^(2+)# has a coordination number of #4#. You can see that if you read this.
It would be #["CoCl"_4]^(2-)#. It IS blue, and it is tetrahedral.
Here is one way #["CoCl"_4]^(2-)# it could form:
#["Co"("H"_2"O")_6]^(2+)(aq) + 4"Cl"^(-)(aq) rightleftharpoons ["CoCl"_4]^(2-) + 6"H"_2"O"(l)#
Reacting with #HCl# shifts the equilibrium to the right. This reaction is endothermic, so heating would favor the products and vice versa.
As for thermodynamic balance, consider the spectrochemical series. Ligands to the right can displace ligands to the left.
Water, a good #sigma# donor and weak #pi# donor, is a stronger-field ligand than chloride, a strong #pi# donor but weak #sigma# donor, so this is not favored towards the products side; excess #"Cl"^(-)# is required.
PRODUCT FROM COOLING
Next, we see that cooling will shift the reaction towards the aqua complex. #["Co"("H"_2"O")_6]^(2+)# exists; it's called hexaaquacobalt(II).
#["Co"("H"_2"O")_6]^(2+)# contains #"Co"^(2+)#, which forms a #d^7# transition metal complex in the context of crystal field theory.
This has #6# electrons in the #t_(2g)# orbitals and #1# in an #e_g# orbital. Since water is a #sigma# donor, contributing a HOMO that is lower in energy than the LUMO of #"Co"^(2+)#, it destabilizes the resultant #e_g# orbitals and contributes antibonding character to them.
Here is an MO diagram of a #d^7# #ML_6# complex, based off of ligand field theory, a more complete version of crystal field theory, so to speak (I added electrons on top of a diagram from Inorganic Chemistry, Miessler et al., pg. 366):
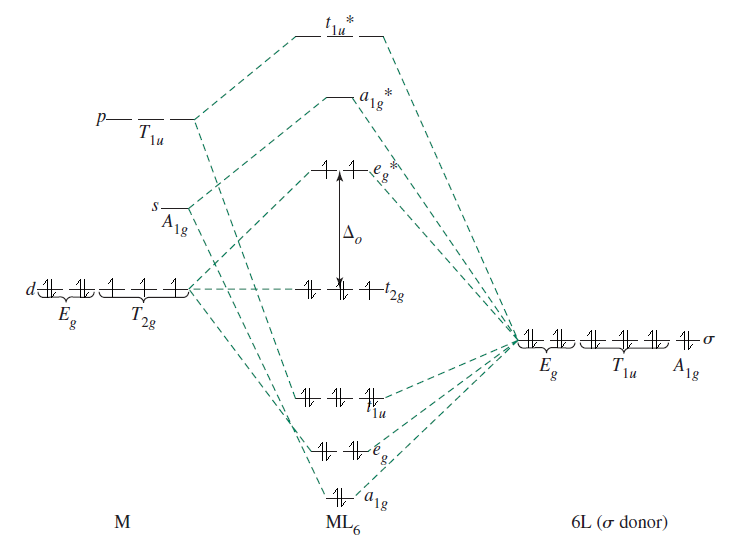
Water is a fairly weak-field ligand, so it produces a high-spin complex, meaning that the first #5# electrons that fill the #t_(2g)# and #e_g^"*"# orbitals are unpaired. The remaining #2# are added to the #t_(2g)# orbitals.
You see how the #E_g# atomic orbitals (the #d_(z^2)# and #d_(x^2 - y^2)#) correspond to the #e_g^"*"# molecular orbitals that are higher in energy than their corresponding atomic orbitals? That's what I mean by antibonding character.
Any oxidation of the metal center in this case is difficult; #2# electrons into the #e_g^"*"# orbitals, which, as I just said, have antibonding character. It means that adding more electrons at this point is unfavorable.
Therefore, no reaction would occur with #"H"_2"O"_2# yet.
REACTION WITH AMMONIA
Fortunately, that could react with #"NH"_3# instead, which is the next step, and is strongly favored in the #"NH"_4^(+)"/""NH"_3# equilibrium (about #27# #"pKa"# units' worth of favorability).
Then you would get #["Co"("NH"_3)_6]^(2+)#. #"NH"_3# is a stronger-field ligand than water, because it is a stronger #sigma# donor. Therefore, it can displace water, sure...
But we're not done here. This is not the final product.
REACTION WITH HYDROGEN PEROXIDE
You CAN use #"H"_2"O"_2# as an oxidizing agent at this point. It would follow roughly the same procedure as outlined here for forming #["Co"("NH"_3)_6]"Cl"_3# from #"CoCl"_2#, an intermediate species in the first equilibrium reaction shown in this answer.
So no, the final product is #["Co"("NH"_3)_6]^(3+)#, not #2+#.

