What is the mass of #NH_4Cl# that must dissolve in 200 grams of water at 50°C to make a saturated solution?
1 Answer
Approximately
Explanation:
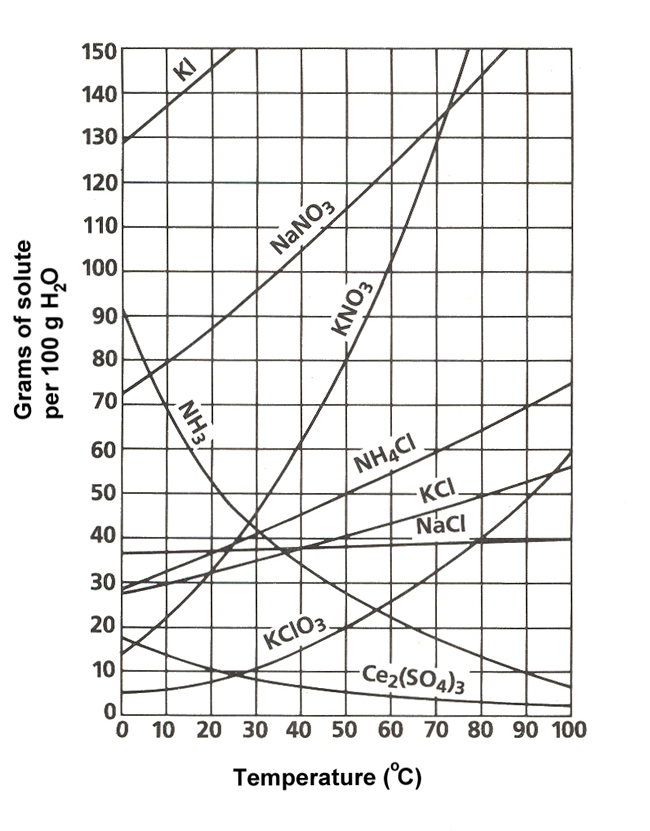
Your tool of choice here will the solubility graph for ammonium chloride,
Before reading the solubility graph, make sure that you have a clear understanding of what it is you're looking for here.
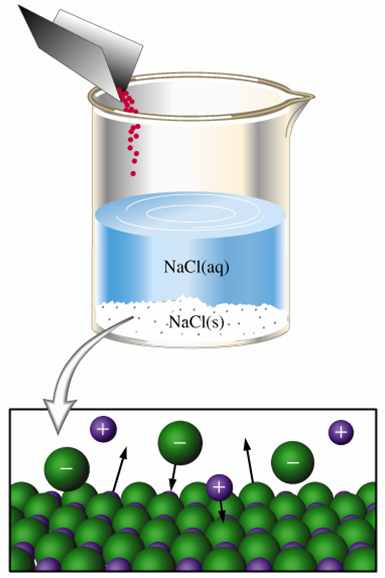
A saturated solution is essentially a solution in which the dissolution and the crystallization of the solute are at equilibrium.
Simply put, the rate at which the ions are dissociated into solution will be equal to the rate at which dissolved ions will recrystallize into the solid.
This means that you're looking for the mass of ammonium chloride that will allow the solution to have the maximum concentration of dissolved ions at that given temperature.
Now, the solubility graph will allow you to find the solubility of the solute per
At
This means that dissolving
Use this value as a conversion factor to determine how much ammonium chloride can be dissolved in
#200color(red)(cancel(color(black)("g water"))) * overbrace(("50 g NH"_4"Cl")/(100color(red)(cancel(color(black)("g water")))))^(color(purple)("solubility at 50"^@"C")) = color(green)(|bar(ul(color(white)(a/a)"100 g NH"_4"Cl"color(white)(a/a)|)))#