Question #1183c
1 Answer
The phenyl cation is not involved in resonance because the vacant orbital is in the plane of the benzene ring.
Explanation:
The carbon atoms in benzene are
They are joined to each other by
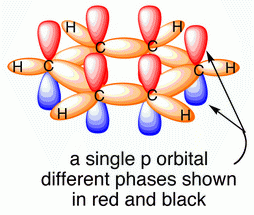
(from www.chemtube3d.com)
The unhybridized
The phenyl cation is formed by removing an
If we draw the phenyl cation as below, it looks as if we could draw curved arrows to get resonance structures.
![]()
But we can't do this!.
Remember that the
The major lobe of the orbital has everywhere a "+" amplitude.
Any overlap of the
There is no net overlap and therefore no resonance with the vacant orbital.
We say that the two orbitals are orthogonal to each other.
NOTE: The phenyl cation still has the normal aromatic resonance, because the π system is unchanged.

