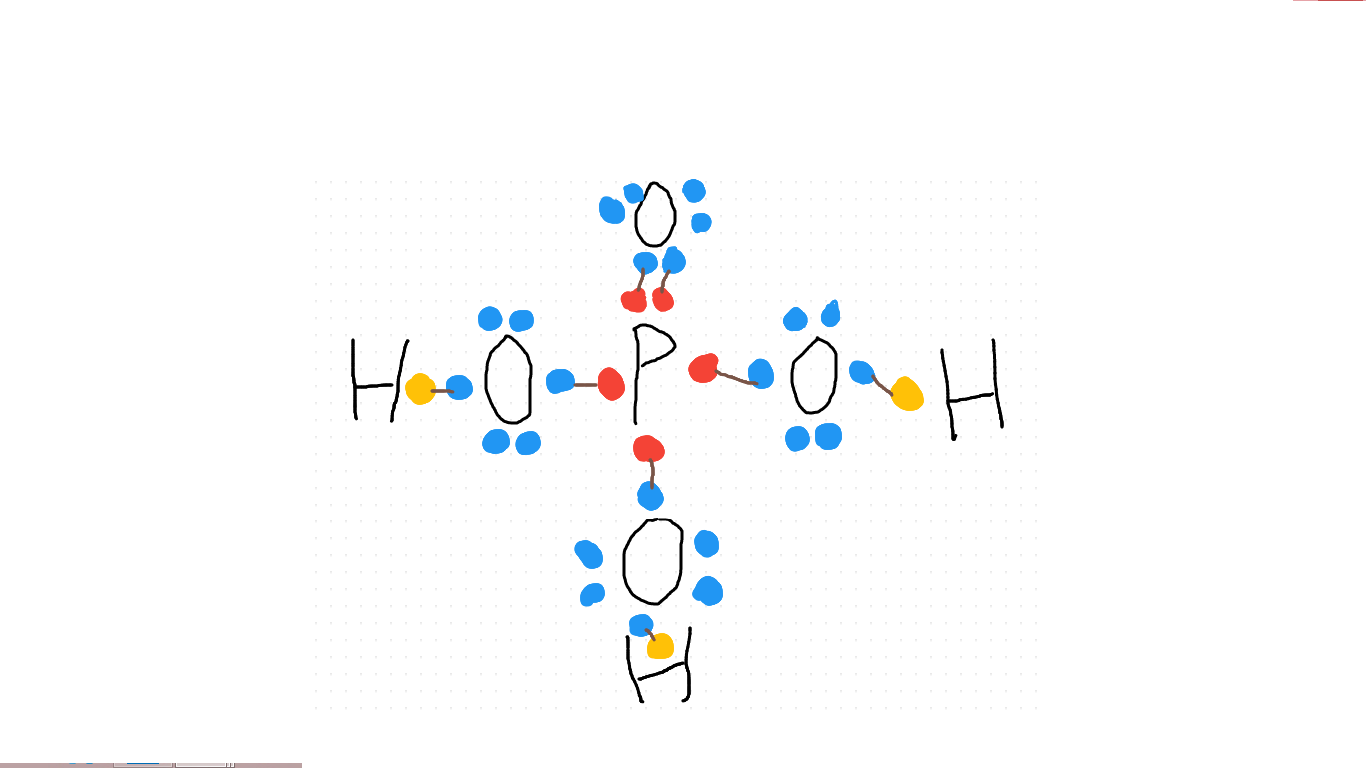
What is the Lewis Dot structure for #H_3PO_4#?
1 Answer
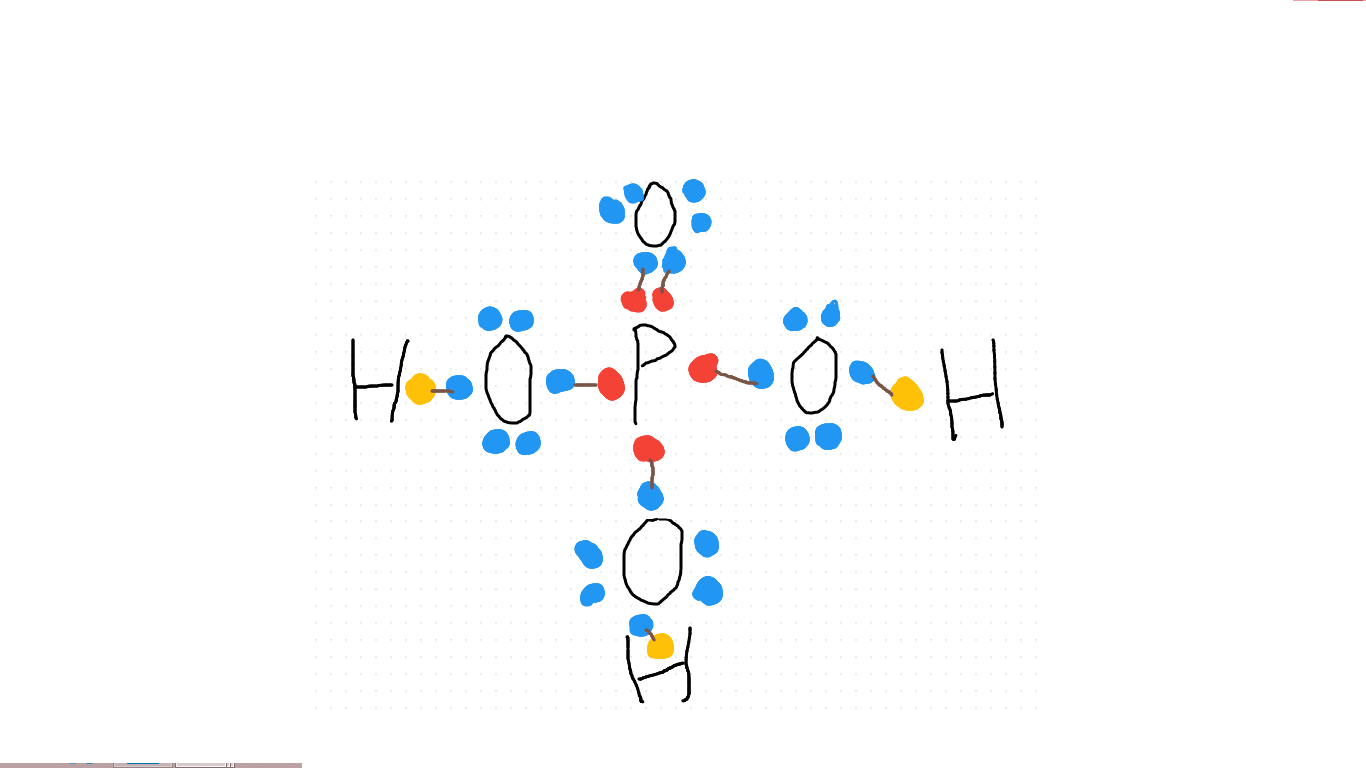
Explanation:
First determine the types of bonds you'd expect by looking at the elements involved and recalling some facts about bonding: covalent between non-metals; ionic between metal and non-metal; and metallic between metals. Covalent is sharing; ionic is giving or taking; metallic is a free-for-all sea of electrons.
Now look for the atomic number of each element involved, which will give you the number of electrons in the outer shell that move around to form bonds. Oxygen has 6 valence
Draw the atoms on: three hydrogen, one phosphorous and four oxygen, and link up electrons until you find none unpaired and no atoms have spaces left in their valence shells. Once every shell is full and every electron partnered, it should look like this: