Question #7bb22
1 Answer
Inter-molecular forces effect boiling point because stronger forces make it a higher boiling point and weaker forces make it a lower boiling point.
Explanation:
Inter-molecular force literally means the force that happens between molecules. There are three types of Inter-molecular forces;
(weakest to strongest)
1. Dispersion forces
2. Dipole forces
3. Hydrogen bonding forces
If you already know what each of these are, skip to below the diagrams.
Dispersion force is the naturally gravity between molecules, causing them to very slightly pull together. Dispersion force happens with every molecule in existence because molecules are matter and matter has mass and all masses has a gravitational pull, even if its a weak one.
Dipole force is the pull between slightly oppositely charged atoms in molecules. Naturally, you rarely get a perfectly balanced balanced molecule where the magnetic strength of each molecule is the same. This causes the electrons shared in the molecule to hang out near one type of atom more than they do the other one, resulting in a minuscule variation in its charge that isn't enough to make them become an ionic molecule, but enough to attract atoms outside the molecule.
Hydrogen bonding forces is the strong force that attracts Hydrogen atoms in a molecule to the atoms Nitrogen, Oxygen, and Fluorine (NOF). It works the same way as Dipole forces do except N, O, and F have such a high electronegativity that it often just results in strongly pulling with the Hydrogen, both of which already in their own compound which is the reason for them not completely breaking off.
A perfect example for all three of these forces is H2O.
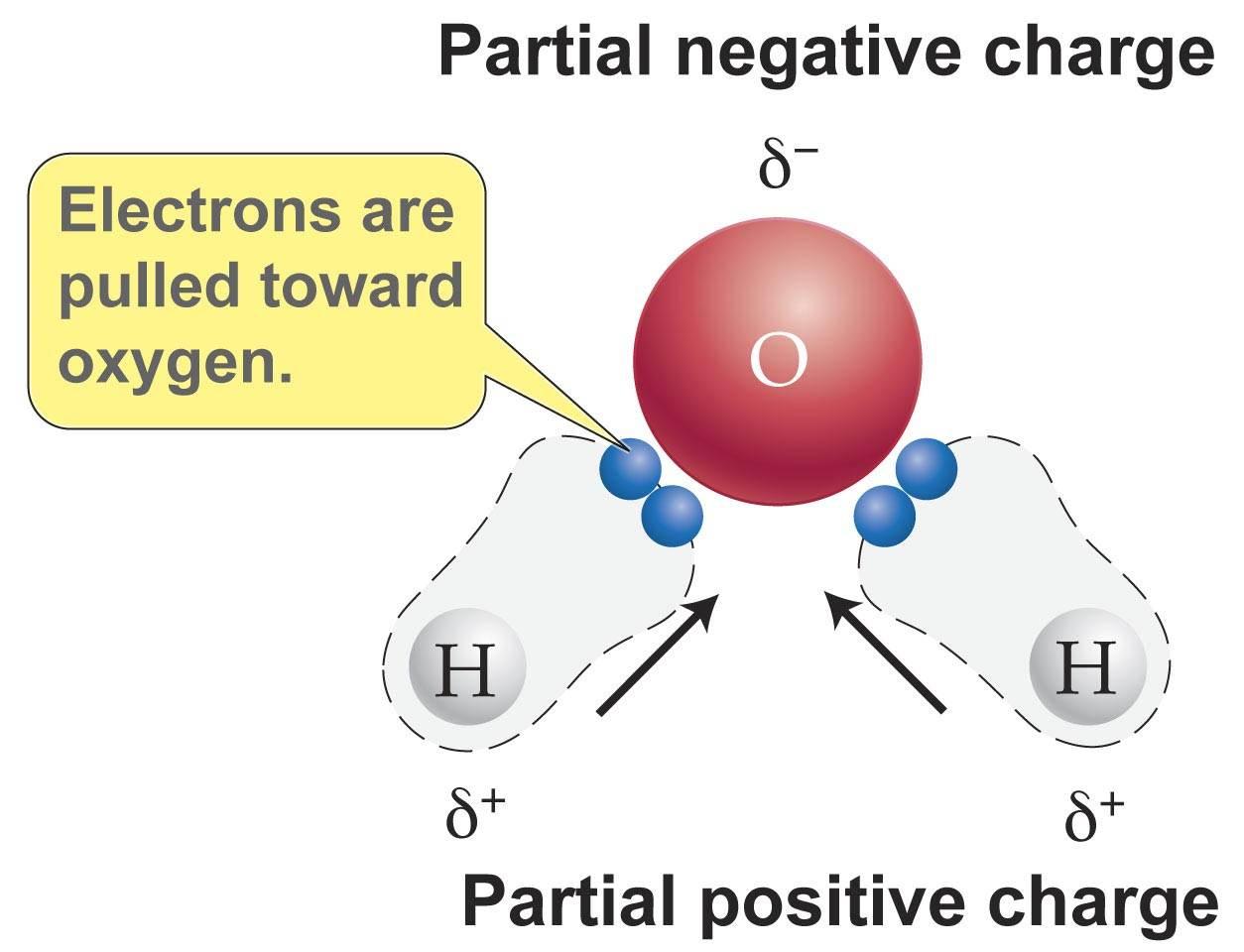
As seen above, the electrons are pulled towards the Oxygen because it has a higher electronegativity resulting in the slightly negative charge.
.
With the dotted lines representing the hydrogen bonds (which is also a type of dipole bond), you can see how the molecules orientate themselves to pull each other closer together in the below diagram. Since the molecules have mass, they are always being affected by dispersion.
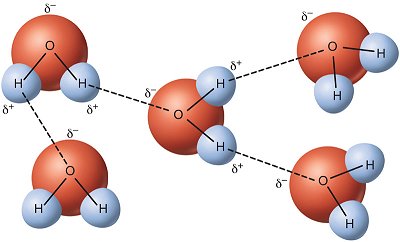
Now to answer you question...
Inter-molecular forces affect boiling point because when something boils it turns into a gas, and part of being a gas is having all the molecules spread further apart. If molecules have a stronger inter-molecular force holding them together, more energy (heat is a form of energy, measured by temperature) is needed to break that force.
You can change boiling point by adding another type of molecule that will interfere with the bonds attracting the molecules together, helping spread out the molecules and become closer to the gaseous state.
One way you can try to display an outside molecule affecting a solutions boiling point is if you take a pot of boiling water then add ALOT of salt into it and stir to dissolve it. if you added enough Salt the water should (nearly) stop boiling, this is for a different yet similar reason that is too long to add to this lesson but basically this experiment displays how new molecules in a solution can affect bonds and therefore boiling point.

