A jeweler has five rings, each weighing 18g, made of 5% silver and 95% gold. He decides to melt down the rings and add enough silver to reduce the gold content to 75%. How much silver should he add?
2 Answers
Detailed explanation
Added silver is 24 grams
Explanation:
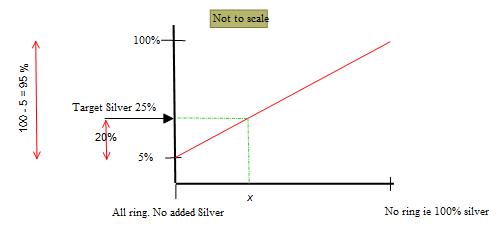
Targeting silver content as the means of determining the blend ratio.
The process is based on two extreme condition:
All ring
No ring
The vertical axis is the silver content of the alloy.
The horizontal axis is the percentage of the added silver.
The target silver content of the alloy is 25%
Turn everything upside down (invert)
Multiply throughout by 20
'~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Not that the fraction is precise. The decimal is not!
So to convert this to the format we require
'~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
This means that the rings represent
Thus the weight of the rings represents
Let the total weight be
Then
'~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Explanation:
Here's an alternative approach to use. You know that each ring contains
#18 color(red)(cancel(color(black)("g ring"))) * "5 g silver"/(100color(red)(cancel(color(black)("g ring")))) = "0.9 g silver"#
This means that the mass of gold will be
#m_"gold" = "18 g" - "0.9 g" = "17.1 g gold"#
Now, you know that the jeweler is working with five rings. The total mass of silver and the total mass of gold present in the five rings will be
#5color(red)(cancel(color(black)("rings"))) * "0.9 silver"/(1color(red)(cancel(color(black)("ring")))) = "4.5 g silver"#
#5 color(red)(cancel(color(black)("rings"))) * "17.1 g gold"/(1color(red)(cancel(color(black)("ring")))) = "85.5 g gold"#
The total mass of the rings will be
#m_"total" = overbrace("4.5 g")^(color(blue)("mass of silver")) + overbrace("85.5 g")^(color(blue)("mass of gold")) = "90 g"#
Now, let's assume that
The mass of gold remains unchanged by the addition of silver. Adding
#overbrace(85.5color(white)(a) color(red)(cancel(color(black)("g"))))^(color(purple)("mass of gold")) = 75/100 * overbrace((90 + x)color(red)(cancel(color(black)("g"))))^(color(purple)("total mass of the mixture"))#
Isolate
#8550 = 6750 + 75x#
#75x = 1800 implies x = 1800/75 = 24#
Therefore, you must add


