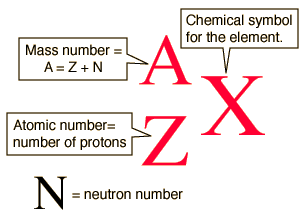Question #b1ea6
1 Answer
Here's what I got.
Explanation:
Isotope notation allows you to identify various isotopes by using atomic number,
So, let's start with antimony-125. Grab a periodic table and look for antimony,
Antimony has an atomic number equal to
Since you know that the given isotope has a mass number equal to
color(green)(|bar(ul(color(white)(a/a)color(black)(""_(color(white)(a)50)^125"Sn")color(white)(a/a)|))) -> antimony-125
Next up, a bismuth nucleus that contains
No,w to get he mass number of this isotope, add the number of protons and the number of neutrons located in the nucleus
A = Z + N
A = 83 + 120 = 203
The isotope notation for this particular isotope will thus be
color(green)(|bar(ul(color(white)(a/a)color(black)(""_(color(white)(a)83)^203"Bi")color(white)(a/a)|))) -> bismuth-203
Finally, a nucleus of element 35 that contains
You'll find that this element is bromine,
Calculate the isotope's mass number by using the number of protons and the number of neutrons it contains in its nucleus
A = 35 + 46 = 81
The isotope notation for this isotope will be
color(green)(|bar(ul(color(white)(a/a)color(black)(""_ 35^81"Br")color(white)(a/a)|))) -> bromine-81