Question #d16b3
1 Answer
Two atoms of hydrogen.
Explanation:
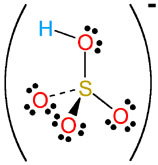
Sulfuric acid,
More specifically, one molecule of sulfuric acid will contain two atoms of hydrogen. These two hydrogen atoms will be bonded to two of the four atoms of oxygen present in the sulfate anion,
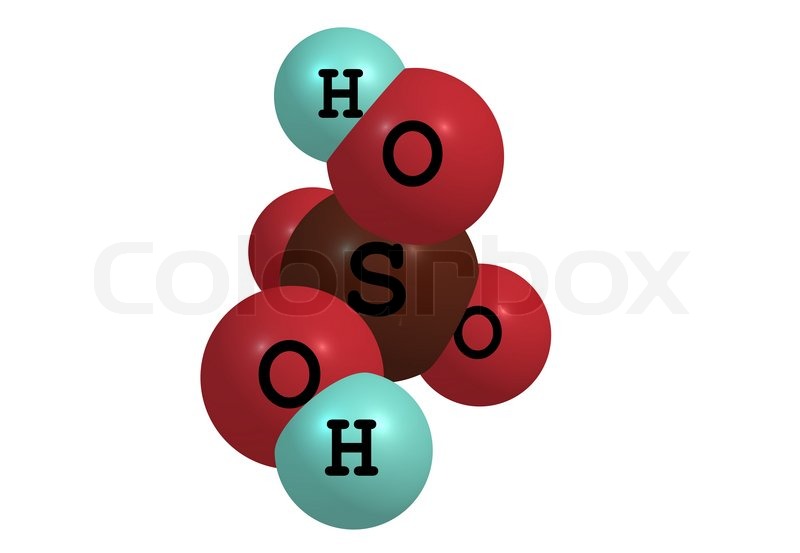
Molecular hydrogen has two hydrogen atoms bonded by a single covalent bond, which as you can see is not the case here.
In aqueous solution, sulfuric acid can donate two protons,
#"H"_ 2"SO"_ (4(aq)) -> "H"_ ((aq))^(+) + overbrace("HSO"_(4(aq))^(-))^(color(blue)("hydrogen sulfate"))#
which looks like this
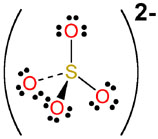
and finally the sulfate anion
#"HSO"_ (4(aq))^(-) rightleftharpoons "H"_ ((aq))^(+) + "SO"_(4(aq))^(2-)#
which looks like this
So, to sum this up, sulfuric acid contains two atoms of hydrogen, not one molecule of hydrogen.