What is the Bronsted-Lowry acid and base versus Lewis?
1 Answer
Here's how I understand it. We will look at some straight definitions, and then we will put them into context right afterwards.
A Brønsted-Lowry acid is a proton donor, while a Lewis base is an electron donor.
When a proton (
If a bond between a Brønsted-Lowry acid and a proton breaks, the Brønsted-Lowry acid donates the proton.
Thus, a Lewis base can donate electrons to acquire a proton, while the Brønsted-Lowry acid had donated that proton. They can work together!
From the other perspective, a Brønsted-Lowry base is a proton acceptor, while a Lewis acid is an electron acceptor.
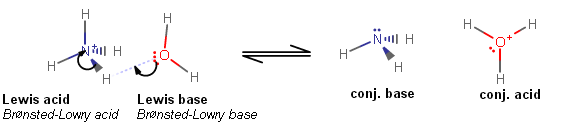
If a Brønsted-Lowry acid (like
Furthermore, if that proton donates itself onto the Lewis base (water in this case), that base is also a Brønsted-Lowry base (water in this case), because it accepted the proton.
Thus, a Lewis acid that possesses a proton can accept electrons to donate a proton, while the Brønsted-Lowry base accepts that proton. Again, they work together!

