Write the reaction which has a heat of reaction equal to heat of formation for HCl(g) ?
Write the reaction which has a heat of reaction equal to heat of formation for HCl(g) ?
Write the reaction which has a heat of reaction equal to heat of formation for HCl(g) ?
1 Answer
Explanation:
A compound's standard heat of formation, or standard enthalpy of formation,
In your case, the chemical reaction that has a change in enthalpy equal to
Now, one molecule of hydrogen chloride contains one atom of hydrogen,
The key now is to remember that hydrogen and chlorine exist as diatomic elements in their standard state.
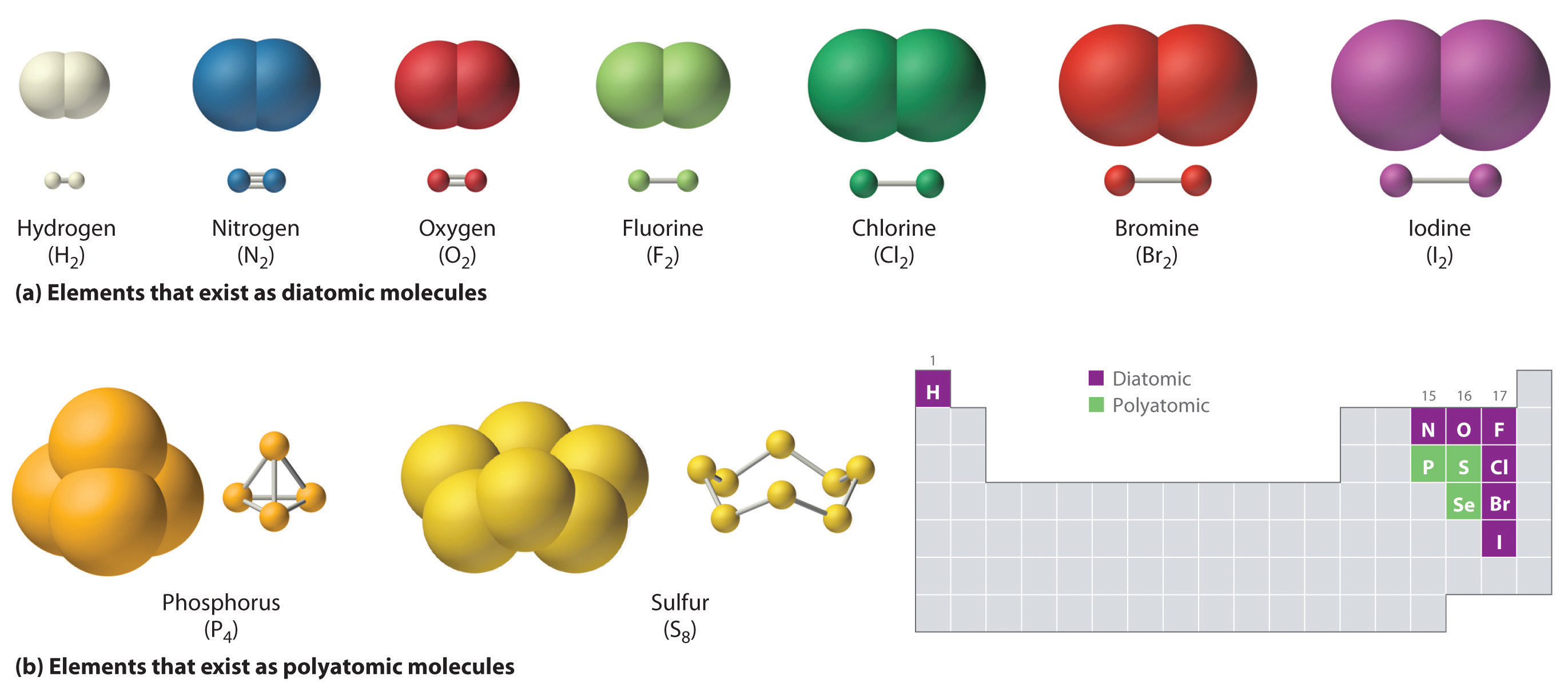
This means that you can write
#"H"_ (2(g)) + "Cl"_ (2(g)) -> 2"HCl"_((g))#
This represents one form of the balanced chemical equation that describes the synthesis of hydrogen chloride.
In order for the enthalpy change of reaction to be equal to that of the standard enthalpy change of formation, you must divide everything by
#color(green)(|bar(ul(color(white)(a/a)color(black)(1/2"H"_ (2(g)) + 1/2"Cl"_ (2(g)) -> "HCl"_((g)))color(white)(a/a)|)))#
This equation will have
#DeltaH_"rxn"^@ = DeltaH_f^@#
because it describes the formation of one mole of hydrogen chloride from its constituent elements in their standard state.

