Question #f3a1e
1 Answer
Explanation:
Start by assigning oxidation numbers to the chemical species involved in the reaction
#stackrel(color(blue)(+1))("Ag"^(+))_ ((aq)) + stackrel(color(blue)(0))("Cu")_ ((s)) -> stackrel(color(blue)(+2))("Cu")""^(2+) ""_ ((aq)) + stackrel(color(blue)(0))("Ag")_ ((s))#
Notice that the oxidation number of silver went from
On the other hand, copper's oxidation number went from
The oxidation half-reaction will look like this
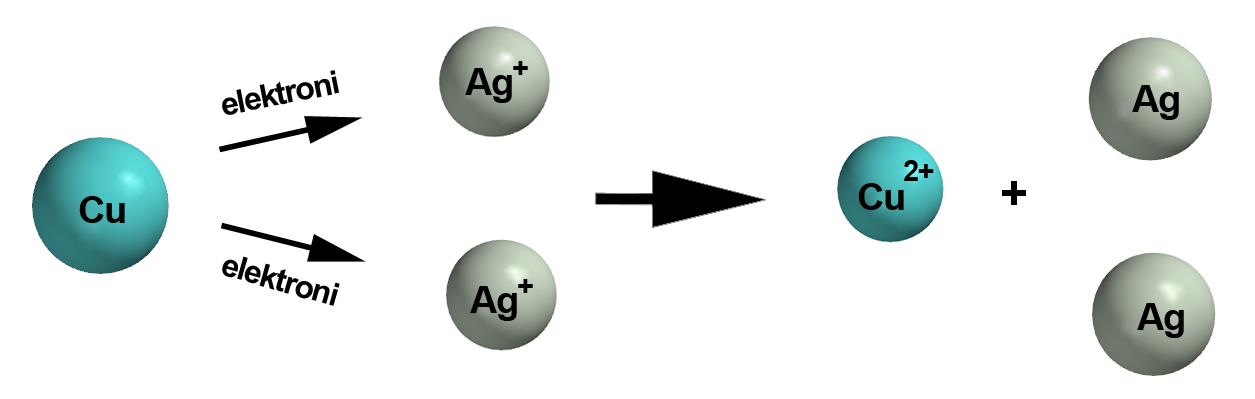
#stackrel(color(blue)(0))("Cu")_ ((s)) -> stackrel(color(blue)(+2))("Cu")""^(2+) ""_ ((aq)) + 2"e"^(-)#
Here each atom of copper is losing two electrons to form the copper(II) cation,
The reduction half-reaction will look like this
#stackrel(color(blue)(+1))("Ag"^(+))_ ((aq)) + "e"^(-) -> stackrel(color(blue)(0))("Ag")_ ((s))#
Here each silver(I) cation is gaining one electron to form a silver atom.
Now, in any redox reaction, the number of electrons gained in the reduction half-reaction must be equal to the number of electrons lost in the oxidation half-reaction.
In your case, you need to multiply the reduction half-reaction by
#{(color(white)(aaaaaaa)stackrel(color(blue)(0))("Cu")_ ((s)) -> stackrel(color(blue)(+2))("Cu")""^(2+) ""_ ((aq)) + 2"e"^(-)), (stackrel(color(blue)(+1))("Ag"^(+))_ ((aq)) + "e"^(-) -> stackrel(color(blue)(0))("Ag")_ ((s)) color(white)(aaaaaaaaaa)| xx 2) :}#
#color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)/(color(white)(aaaaaaaaaaaaaa)#
#"Cu"_ ((s)) + 2"Ag"_ ((aq))^(+) + color(red)(cancel(color(black)(2"e"^(-)))) -> "Cu"_ ((aq))^(2+) + color(red)(cancel(color(black)(2"e"^(-)))) + 2"Ag"_((s))#
The balanced chemical equation will thus be
#color(green)(|bar(ul(color(white)(a/a)color(black)("Cu"_ ((s)) + 2"Ag"_ ((aq))^(+) -> "Cu"_ ((aq))^(2+) + 2"Ag"_((s)))color(white)(a/a)|)))#
In this reaction, copper acts as a reducing agent because it reduces silver(I) cations to silver metal. Likewise, the silver(I) cations act as an oxidizing agent because they oxidize copper metal to copper(II) cations.