How does specific heat relate to how fast a substance changes temperature?
1 Answer
I can explain it as follows
Explanation:
We know that the amount of heat gain or loss
#DeltaH=mxxsxxDeltat#
It is clear from the above equation that if the mass 'm' and the amount of heat gain or loss(
So
#Deltat prop 1/s#
From this relation we can easily conclude that the amount of rise or fall of temperature is higher or lower accordingly as the sp.heat of the object is lower or higher.
Example: Let us discuss an interesting natural phenomenon associated with it.
Due to higher sp.heat of water as compared to average sp.heat of land mass the flow of sea breeze at day time and flow of land breeze at night are found to occur near coastal region.
In day time the water part and land part of earth receives heat at the same rate. But the sp.heat of water being much greater than land mass the land part gets warmer quickly and it makes the air over land warmer quickly. This lowers the density of air over land mass and that is why the air over land gets an upward movement . At that time comparatively cold air from water part flows towards land and this flow of air from sea to land at day time is known as sea breeze.
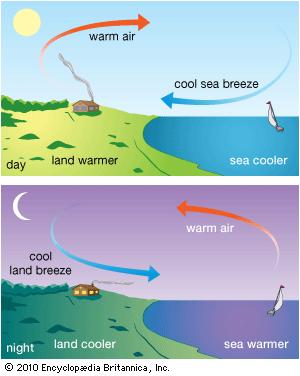
At night The earth radiates its heat energy. But the sp.heat of land mass being very low as compared to water it loses its temperature at higher rate than water part and as a result the water remains more hotter than land part at night. The air over water part also remains hotter and it gains upward movement due to its low density. At that time comparatively cold air over land part flows towards sea and this flow of air from land to sea at night is known as land breeze

