Explain London dispersion forces with an analogy?
1 Answer
London dispersion forces, or "London forces", arise after a molecule (or atom) and some other nonpolar molecule (or atom) interact, distorting the electron cloud of the nonpolar molecule/atom and temporarily polarizing it.
Let's focus on atoms for simplicity.
ANALOGY: BUBBLES
An OK analogy is when you have two bubbles floating in midair, and one of them flies into the other. This disturbs the equilibrium of the second bubble, and it starts jiggling.

If this second bubble then flies into a third bubble, that third bubble gets disturbed as well.
DISTURBING THE ELECTRON CLOUD
This analogy ties back into the idea of an atom weakly attracted to a nonpolar atom, approaching it, and disturbing its electron cloud after coming near it. This "jiggles" the electron cloud in the atom, polarizing it.
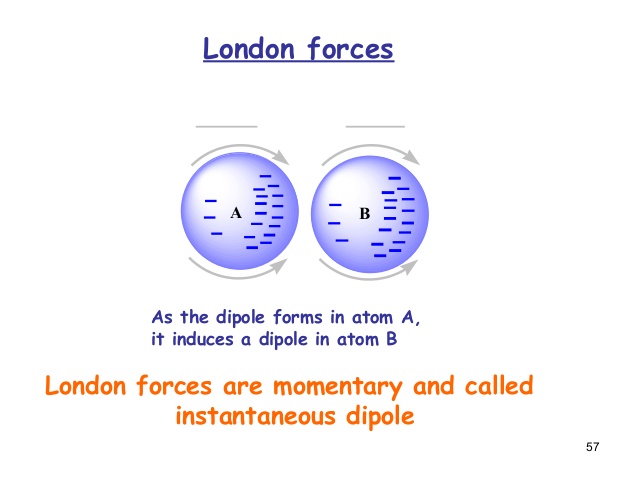
Now, the charge density is unevenly distributed in the atom.
AFTER DISTURBING THE ELECTRON CLOUD?
The region of negative charge (the electron density) in the atom then moves towards the region of positive charge in another undisturbed atom.
The electron density in THAT atom repulsively moves away from the incoming electron density (negative repels negative).
#stackrel("atom 1")overbrace(delta^(+) " " delta^(-)) -> stackrel("atom 2")overbrace(color(red)(delta^(+) delta^(-))#
#=>stackrel("atom 1")overbrace(delta^(+) " " delta^(-)) stackrel("atom 2")overbrace(color(red)(delta^(+) " "" "delta^(-)))#
Now, the charge density is unevenly distributed in THAT atom as well.
(kind of like pool balls colliding in a chain reaction!)
CONCLUSIONS
Overall, when the electron density in an atom comes close to a region of positive charge on another undisturbed atom, it gets weakly attracted to that region of positive charge and disturbs the charge distribution on that atom, polarizing that atom as well.
This weak attraction that is temporarily-induced is a London dispersion force, an "intermolecular force" (or I guess "interatomic" in this case).
INTERPRETATIONS
Larger atoms can get polarized more easily, because the electrons are farther away from the nucleus on average, experiencing less attraction to the positively-charged nucleus (therefore more freely-moving).
So, larger atoms/molecules will generally experience stronger London dispersion forces.
For instance, those forces are stronger for

