Question #8b34f
1 Answer
About
Explanation:
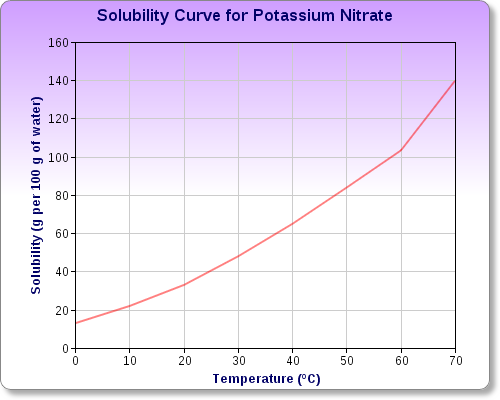
Potassium nitrate,
You can see this by examining potassium nitrate's solubility curve, which looks like this
 http://www.sciencequiz.net/jcscience/jcchemistry/water_solutions/water_solutions_mcq.htm
http://www.sciencequiz.net/jcscience/jcchemistry/water_solutions/water_solutions_mcq.htm
As you can see, you can dissolve
In other words, dissolving that much potassium nitrate in
Any excess of potassium nitrate added over the solubility of the salt will remain undissolved.
Notice that as temperature increases, the solubility of the salt increases as well.

