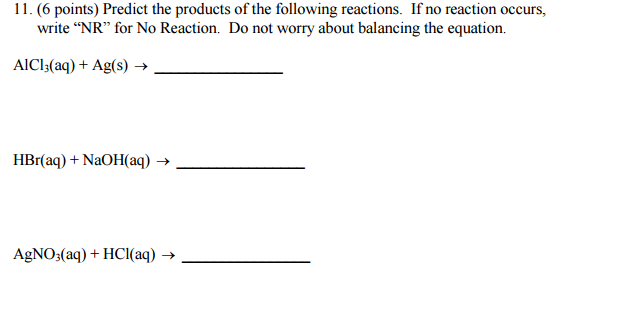
Predict the products of the following reactions. If no reaction occurs, write “NR” for No Reaction. Do not worry about balancing the equation?
1 Answer
Here's what I got.
Explanation:
You can predict if single replacement reactions will take place by using the metal reactivity series.
In general, a metal that is higher in the reactivity series will displace a metal that is lower in the reactivity series.
In your case, aluminium,
Therefore, you will have
#"AlCl"_ (3(aq)) + "Ag"_ ((s)) -> "N.R."#
The second reaction will take place because hydrobromic acid,
#"HBr"_ ((aq)) + "NaOH"_ ((aq)) -> "NaBr"_ ((aq)) + "H"_ 2"O"_ ((l))#
In this case, you're dealing with a neutralization reaction that basically looks like this
#overbrace("H"_ 3"O"_ ((aq))^(+))^(color(blue)("coming from HBr")) + overbrace("OH"_ ((aq))^(-))^(color(green)("coming from NaOH")) = 2"H"_ 2"O"_((l))#
The third reaction will take place because it results in the formation of silver chloride,
#"AgNO"_ (3(aq)) + "HCl"_ ((aq)) -> "AgCl"_ ((s)) darr + "HNO"_ (3(aq))#
This is a double replacement reaction for which the net ionic equation looks like this
#"Ag"_ ((aq))^(+) + "Cl"_ ((aq))^(-) -> "AgCl"_ ((s)) darr#
Silver chloride is a white insoluble solid that precipitates out of solution.



