How would you determine the ionization energy of a hydrogen atom (in kJ/mol) if the electron is in its ground state?
1 Answer
I will suggest two ways you could do this.
Explanation:
Use the Rydberg expression:
The wavelength
The energy levels in hydrogen converge and coalesce:

Since the electron is in the
You can see that as the value of
The Rydberg expression now becomes:
Since
We can now find the frequency and hence the corresponding energy:
Now we can use the Planck expression:
This is the energy needed to remove 1 electron from 1 hydrogen atom. To find the energy required to ionise 1 mole of H atoms we multiply by the Avogadro Constant:
Use the convergence limit of the u.v spectrum in an experimental method.
You can see from the diagram that the energy levels converge and coalesce to a continuum. This means that the emission lines will converge also.
The frequency at which this happens can give us the ionisation energy.
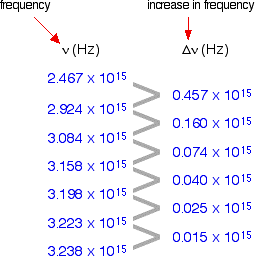
The 1st column shows the frequency of the line.
The second column gives the difference in frequency which is getting less and less.
If you plot
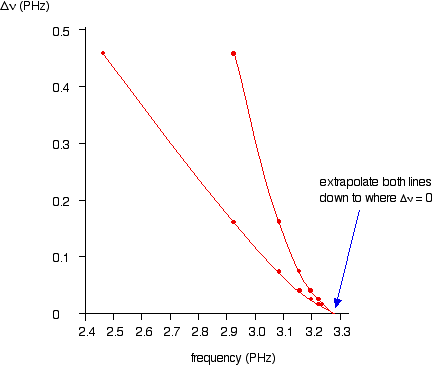
You can actually get 2 lines using upper and lower values. The important point is that they converge to the same point where
You can the read the convergence limit off the x axis.
This gives
As in method
So for 1 mole:

