What is the bond order of #Cl_2 ^-#?
1 Answer
The bond order is 0.5.
Explanation:
We have to construct the molecular orbitals for the
The electron configuration of
When we form
We have 7 electrons from each
Hence we have 15 electrons to assign to the molecular orbitals.
We will put 4 electrons in the
Their contribution to the bonding is zero.
The order of the
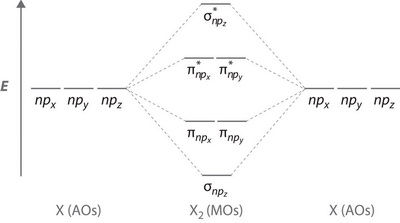
(from 2012books.lardbucket.org)
We assign the 11 remaining electrons to these orbitals as follows:
The bond order is half the number of bonding electrons - half the number of antibonding electrons:
#color(blue)(|bar(ul(color(white)(a/a) "BO" = (B-AB)/2color(white)(a/a)|)))" "#


