What is the total number of grams of #NaI# needed to make 1.0 liter of a 0.010 M solution?
1 Answer
1.50g of NaI
Explanation:
Molarity is represented by the following equation:
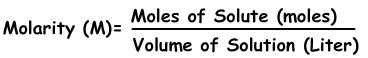
In our case, we already have the molarity and the volume of solution, both of which have the appropriate units.
Now we can rearrange the equation to solve for the number of moles, which will allow us to determine the mass. We can do this by multiplying by liters of solution on both sides of the equation. The liters of solution will cancel out on the right side, leaving the number of moles being equal to the molarity times volume like so:
Moles of solute =
Moles of solute = (1.0 L)
Now we have to convert the 0.010 moles of NaI into grams of NaI. This can be done by multiplying 0.010 moles by the molecular weight of NaI, which is

