Is hyponitrite polar or nonpolar?
1 Answer
...Both (but not at the same time).
The hyponitrite ion,
But it also has a cis major resonance structure that looks like this:
Either way, oxygen is more electronegative than nitrogen, so there are two dipole moment vectors whose components will add to form a net dipole.
-
Here, the two dipole moment vectors cancel out in the horizontal direction (along the
#"N"="N"# bond), but add in the vertical direction (perpendicular to the#"N"="N"# bond), giving a nonzero net dipole.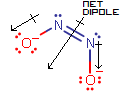
-
Here, the two dipole moment vectors cancel out in both the horizontal (along the
#"N"="N"# bond) and vertical (perpendicular to the#"N"="N"# bond) directions, giving no net dipole.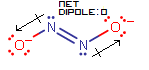
Therefore, the cis isomer is polar, and the trans isomer is nonpolar.

