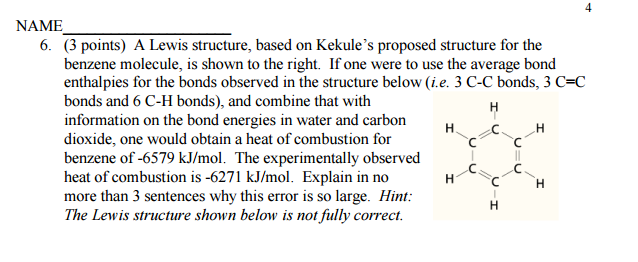
A Lewis structure, based on Kekule’s proposed structure for the benzene molecule, is shown to the right. Explain in no more than 3 sentences why this error is so large?
1 Answer
The magnitude of the enthalpy of combustion depends on the amount of potential energy stored in each bond within the ring.
Due to the pi electron delocalization in the aromatic system, benzene has evenly distributed its energy throughout, which effectively lowers the enthalpy of each pure
Hence, the magnitude of the enthalpy of combustion is substantially smaller.
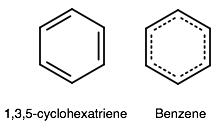
This aromatic stabilization (resonance) is actually significant. We can account for the large difference from looking at the heat of hydrogenation of the theoretical and the actual benzene.
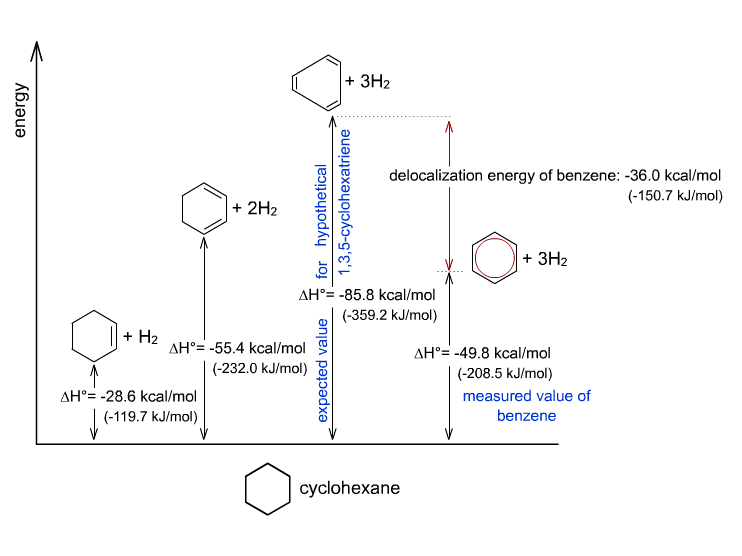
Based on:
- the theoretical enthalpy due to removing the three
#pi# bonds of the hypothetical 1,3,5-cyclohexatriene - the experimental enthalpy due to actually hydrogenating benzene
...the energy due to the
And that's only due to the way the

