If given 4.5 mL of 0.05 M magnesium sulfate, how many moles of magnesium sulfate do we have?
1 Answer
We have
Explanation:
Molarity is represented by the following equation:
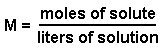
We know the molarity and the volume of solution. However, the volume does not have the proper units since it is given in terms of milliliters instead of liters. We can convert 4.5 mL into liters by using the conversion factor 1000mL = 1L.
When 4.5 mL is divided by 1000 mL/L, we obtain a volume of 0.0045 L.
Now we can rearrange the equation to solve for the number of moles. This can be accomplished by multiplying by liters of solution on both sides of the equation. The liters of solution will cancel out on the right side, leaving the number of moles being equal to the molarity times volume like so:
Moles of solute =
Moles of solute =

