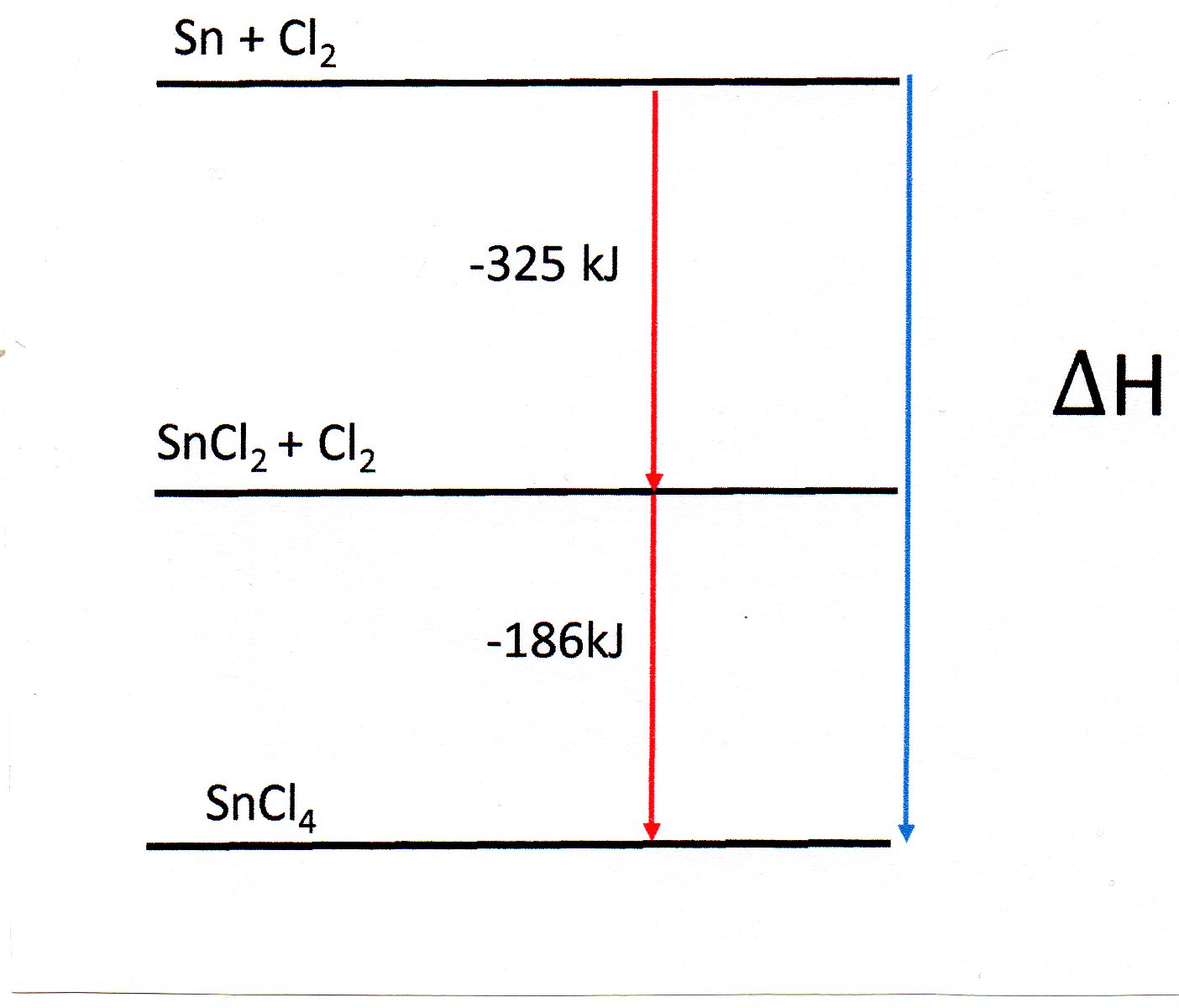
You are given the following two equations. How do you calculate #DeltaH# for this reaction: #Sn(s) + 2Cl_2(g) -> SnCl_4 (l)#?
#Sn(s) + Cl_2(g) -> SnCl_2(s)#
#DeltaH = -325 kJ #
#SnCl_2(s) +Cl_2 (g) -> SnCl_4(l)#
#DeltaH = -186 kJ#
1 Answer
Jul 16, 2016
You can do it like this: