What is the equivalent pressure of 0.743 atm in units of mm Hg?
1 Answer
Explanation:
The problem wants you to use a known conversion factor to go from pressure expressed in atmospheres,
Now, you can find the conversion factor that takes you from atmospheres to millimeter of mercury by using the pascals,
In terms of pascals, an atmosphere is defined as a pressure of
#"1 atm " = " 101325 Pa"#
Similarly, a millimeter of mercury, which is simply the pressure exerted by a
#"1 mmHg " ~~ " 133.32239 Pa"# .
Your goal now is to find how many milliliters of mercury are needed to get to the equivalent of one atmosphere. You can say that you have
#overbrace(101325 color(red)(cancel(color(black)("Pa"))))^(color(blue)("= 1 atm")) * "1 mmHg"/(133.32239color(red)(cancel(color(black)("Pa")))) ~~ "760 mmHg"#
This tells you that a column of mercury
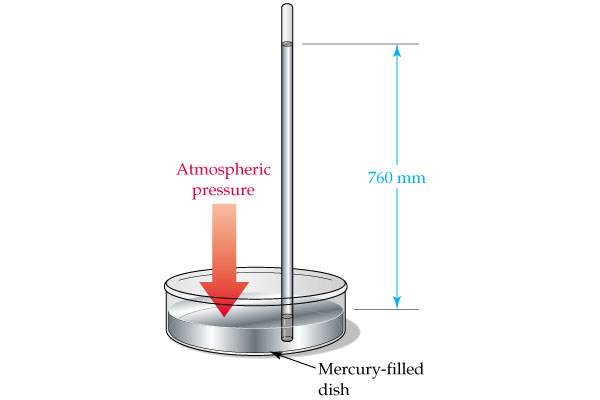
You can thus say that your conversion factor looks like this
#color(purple)(|bar(ul(color(white)(a/a)color(black)("1 atm " = " 760 mmHg")color(white)(a/a)|)))#
Use this conversion factor to find the equivalent pressure of
#0.743 color(red)(cancel(color(black)("atm"))) * "760 mmHg"/(1color(red)(cancel(color(black)("atm")))) = color(green)(|bar(ul(color(white)(a/a)color(black)("565 mmHg")color(white)(a/a)|)))#
The answer is rounded to three sig figs.

