What is the volume occupied by #3.0*10^23# molecules of bromine gas at STP?
1 Answer
Explanation:
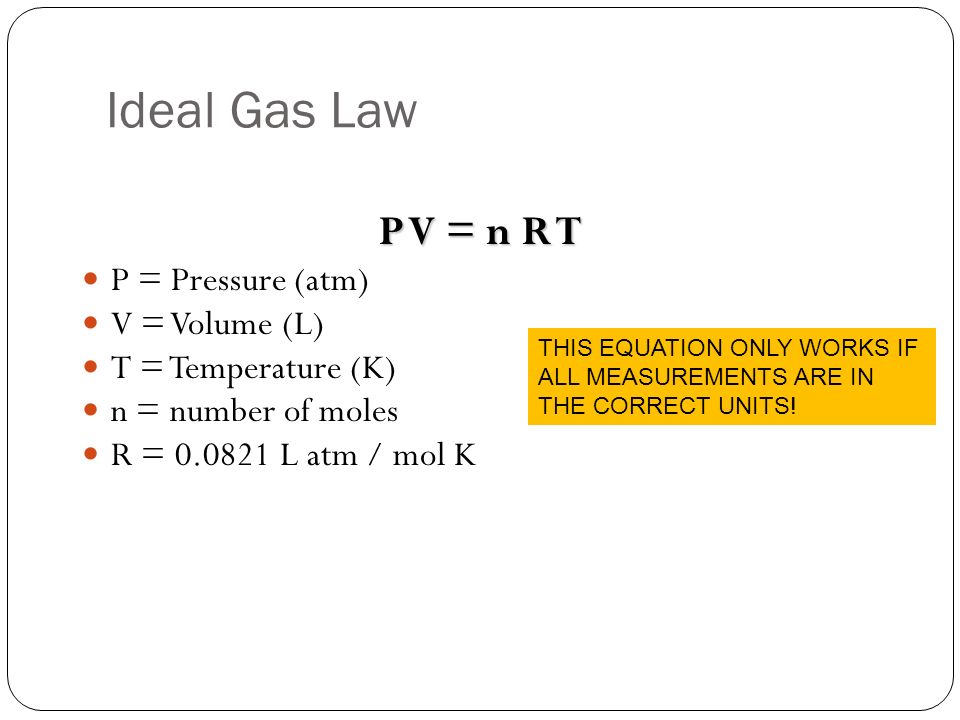
At standard temperature and pressure, the temperature is 273K and the pressure is 1 atm.
Next, list your known and unknown variables. Our only unknown is the volume of
We don't necessarily have 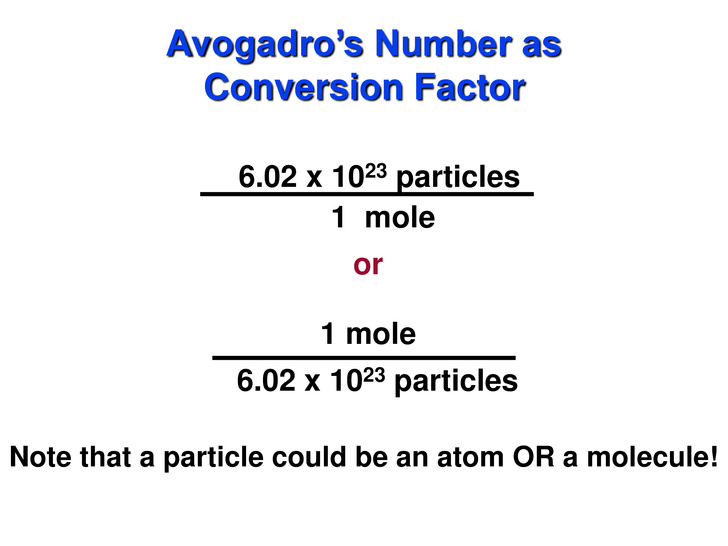
We'll use the second conversion factor because we can cancel out molecules and end up with moles:
Now all of the variables have good units! All that's left to do is rearrange the equation and solve for V like so:

