Question #50238
1 Answer
It is a reduction reaction that plays an important role in cellular respiration.
Explanation:
Gaining electrons
The conversion of
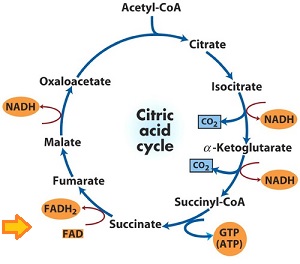
As you can see, a similar thing happens to
Losing electrons
Of course this carrying of electrons has a purpose. The electrons are used in the electron transport chain of cellular respiration. The final goal of the process is to generate ATP, which is the main energy source for all cellular processes.
This transport chain that finally produces ATP requires electrons, The electrons are provided by the

